a. How many grams of Xe are present in 4.35 grams of xenon trioxide? g Xe b. How many grams of xenon trioxide contain 3.42 grams of O? Mass= g xenon trioxide Mass= Use the References to access important values if needed for this question. Submit Answer Retry Entire Group 7 more group attempts remaining
a. How many grams of Xe are present in 4.35 grams of xenon trioxide? g Xe b. How many grams of xenon trioxide contain 3.42 grams of O? Mass= g xenon trioxide Mass= Use the References to access important values if needed for this question. Submit Answer Retry Entire Group 7 more group attempts remaining
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.10: What's In A Mole?: Molar Mass
Problem 8E
Related questions
Question
How Can I solve this ?

Transcribed Image Text:a. How many grams of Xe are present in 4.35 grams of xenon trioxide?
g Xe
b. How many grams of xenon trioxide contain 3.42 grams of O?
Mass=
g xenon trioxide
Mass=
Use the References to access important values if needed for this question.
Submit Answer
Retry Entire Group 7 more group attempts remaining

Transcribed Image Text:1. How many grams of Ag+ are present in 2.22 grams of silver acetate?
grams Ag+.
2. How many grams of silver acetate contain 2.08 grams of Ag+?
grams silver acetate.
Submit Answer
Retry Entire Group
7 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
How would I solve for this one then?
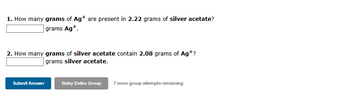
Transcribed Image Text:1. How many grams of Ag+ are present in 2.22 grams of silver acetate?
grams Ag+.
2. How many grams of silver acetate contain 2.08 grams of Ag+?
grams silver acetate.
Submit Answer
Retry Entire Group
7 more group attempts remaining
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER