2) Draw the isobaric phase diagram at 1 atm of the following two-component liquid system: water (Boiling point = 100°C) and nitric acid (Boiling point = 86°C), with the mass percentage of nitric acid on the x-axis. When the liquid mixture contains 68 % nitric acid, an azeotrope with a boiling point of 120.5°C, is formed. Use the sketch to describe under which conditions pure nitric acid can be obtained by means of isobaric distillation.
2) Draw the isobaric phase diagram at 1 atm of the following two-component liquid system: water (Boiling point = 100°C) and nitric acid (Boiling point = 86°C), with the mass percentage of nitric acid on the x-axis. When the liquid mixture contains 68 % nitric acid, an azeotrope with a boiling point of 120.5°C, is formed. Use the sketch to describe under which conditions pure nitric acid can be obtained by means of isobaric distillation.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
100%
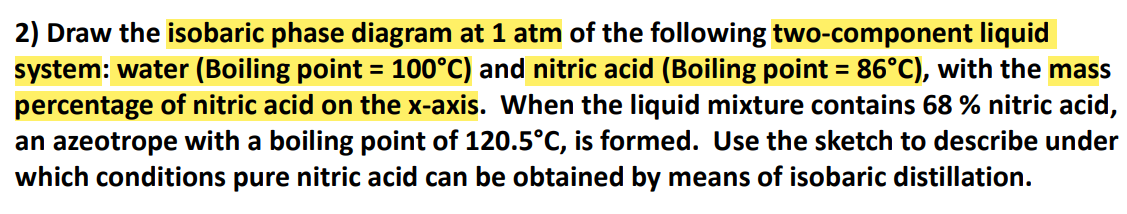
Transcribed Image Text:2) Draw the isobaric phase diagram at 1 atm of the following two-component liquid
system: water (Boiling point = 100°C) and nitric acid (Boiling point = 86°C), with the mass
percentage of nitric acid on the x-axis. When the liquid mixture contains 68 % nitric acid,
an azeotrope with a boiling point of 120.5°C, is formed. Use the sketch to describe under
which conditions pure nitric acid can be obtained by means of isobaric distillation.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning