2. The rovibrational absorption spectrum below corresponds to the V4 band of NH3. Use the wavenumbers of the visible lines in the zoomed region to determine the distance (in Å) of the hydrogen atoms from the principal (C3) axis. Note the lines are colour coded for AK. mN = 14.007 amu, m₁ = 1.008 amu. Q Th 1400 HP ▬ R- 1500 1600 1700 1800 v/cm¹ ΔΚ = 0 AK = ±1 1623.75- 1627.00- 1630.25- 1636.44 1642.64- 1645.89- 1649.14- 1655.03- 1661.52 1664.78- v/cm²¹
2. The rovibrational absorption spectrum below corresponds to the V4 band of NH3. Use the wavenumbers of the visible lines in the zoomed region to determine the distance (in Å) of the hydrogen atoms from the principal (C3) axis. Note the lines are colour coded for AK. mN = 14.007 amu, m₁ = 1.008 amu. Q Th 1400 HP ▬ R- 1500 1600 1700 1800 v/cm¹ ΔΚ = 0 AK = ±1 1623.75- 1627.00- 1630.25- 1636.44 1642.64- 1645.89- 1649.14- 1655.03- 1661.52 1664.78- v/cm²¹
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 29AP
Related questions
Question
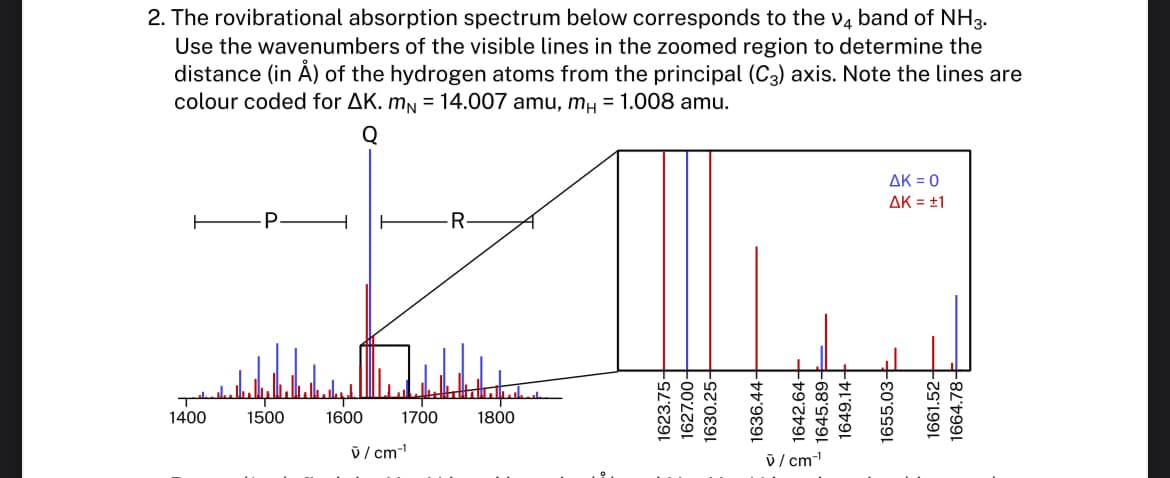
Transcribed Image Text:2. The rovibrational absorption spectrum below corresponds to the V4 band of NH3.
Use the wavenumbers of the visible lines in the zoomed region to determine the
distance (in Å) of the hydrogen atoms from the principal (C3) axis. Note the lines are
colour coded for AK. mN = 14.007 amu, m₁ = 1.008 amu.
Q
-R
1400
1500
1600
1700
1800
V/cm-1
AK = 0
AK = ±1
1623.75-
1627.00-
1630.25-
1636.44-
1642.64-
1649.14-
1645.89
1655.03-
1661.52-
1664.78-
v/cm-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 24 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning