A chemistry student needs 15.0 g of 2-nitroethanol for an experiment. He has available 1.0 kg of a 37.4% w/w solution of 2-nitroethanol in water. Calculate the mass of solution the student should use. Be sure your answer has the correct number of significant digits.
A chemistry student needs 15.0 g of 2-nitroethanol for an experiment. He has available 1.0 kg of a 37.4% w/w solution of 2-nitroethanol in water. Calculate the mass of solution the student should use. Be sure your answer has the correct number of significant digits.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section13.7: Colligative Properties Of Solutions
Problem 13.16E: Suppose that you are closing a cabin in the north woods for the winter and you do not want the water...
Related questions
Question

Transcribed Image Text:A chemistry student needs 15.0 g of 2-nitroethanol for an experiment. He has available 1.0 kg of a 37.4% w/w solution of 2-nitroethanol in water.
Calculate the mass of solution the student should 'use.
Be sure your answer has the correct number of significant digits.
Expert Solution
Step 1
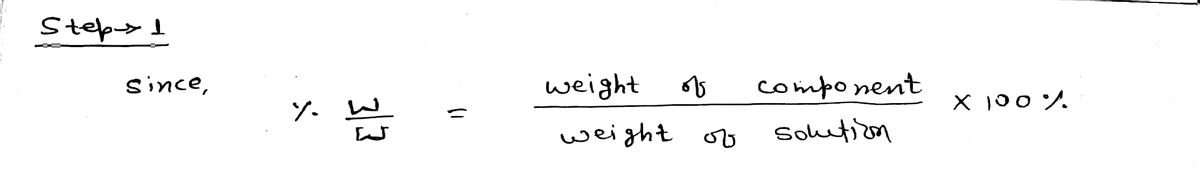
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning