A. Calibration of pH probe (mark with check) B. Titration of HCI 1. M of NaOH (from bottle): 2. Volume of HCI: 25 0.100 pH = 4.0 D pH = 7.0 E pH=10.0 M mL = 0, 025 L == 3. moles of HCI (MHCI X VHCI, in L) = 4. Volume of NaOH at equivalence point: 2.5 moles 0.1/75 mL = L 5. M of NaOH (experimental) at equivalence point (moles NaOH = moles HCl(1/VolNaOH) = 6. pH at equivalence point: Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading 0.00 l 1.00 1.60 2.00 اما ١٠ 16.00 2.0 26.50 10.15 17.00 2.08 27.00 0.97 18.00 2.13 27.50 11.15 3.00 42 19.00 2,20 28.00 11.24 4.00 20.00 1.64 2.27 28.50 1.34 5.00 20.50 2.32 29.00 11.42 6.00 1.69 21.00 2.31 29.00 7.00 8.00 1.71 11:72 21.50 2.43 30.00 11.53 22.00 31.00 2.51 9.00 1.76 22.50 2.59 32.00 11.66 10.00 1.78 23.00 2.7 34.00 11.79 11.00 81 23.50 2.81 36.00 11.86 12.00 11.85 24.00 2.99 38.00 11.94 13 42.50 1.88 24.50 3.29 40.00 12.00 13.00 1.92 25.00 4.30 42.00 12.03 14.00 1.97 25.50 7,21 45.00 12,10 15.00 2.00 26.00 0.17 50.00 127
A. Calibration of pH probe (mark with check) B. Titration of HCI 1. M of NaOH (from bottle): 2. Volume of HCI: 25 0.100 pH = 4.0 D pH = 7.0 E pH=10.0 M mL = 0, 025 L == 3. moles of HCI (MHCI X VHCI, in L) = 4. Volume of NaOH at equivalence point: 2.5 moles 0.1/75 mL = L 5. M of NaOH (experimental) at equivalence point (moles NaOH = moles HCl(1/VolNaOH) = 6. pH at equivalence point: Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading 0.00 l 1.00 1.60 2.00 اما ١٠ 16.00 2.0 26.50 10.15 17.00 2.08 27.00 0.97 18.00 2.13 27.50 11.15 3.00 42 19.00 2,20 28.00 11.24 4.00 20.00 1.64 2.27 28.50 1.34 5.00 20.50 2.32 29.00 11.42 6.00 1.69 21.00 2.31 29.00 7.00 8.00 1.71 11:72 21.50 2.43 30.00 11.53 22.00 31.00 2.51 9.00 1.76 22.50 2.59 32.00 11.66 10.00 1.78 23.00 2.7 34.00 11.79 11.00 81 23.50 2.81 36.00 11.86 12.00 11.85 24.00 2.99 38.00 11.94 13 42.50 1.88 24.50 3.29 40.00 12.00 13.00 1.92 25.00 4.30 42.00 12.03 14.00 1.97 25.50 7,21 45.00 12,10 15.00 2.00 26.00 0.17 50.00 127
Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.29QAP
Related questions
Question
What is caculated pH at 0.00 mL of NaOH and Percentage error? Experiement pH 1.61
What is caculated pH at 12.50 mL of NaOH and Percentage error? Experiement pH is 1.85
What is caculated pH at 25.00 mL of NaOH and Percentage error? Experiement pH is 4.30
What is caculated pH at 30.00 mL of NaOH and Percentage error? Experiement pH is 11.53
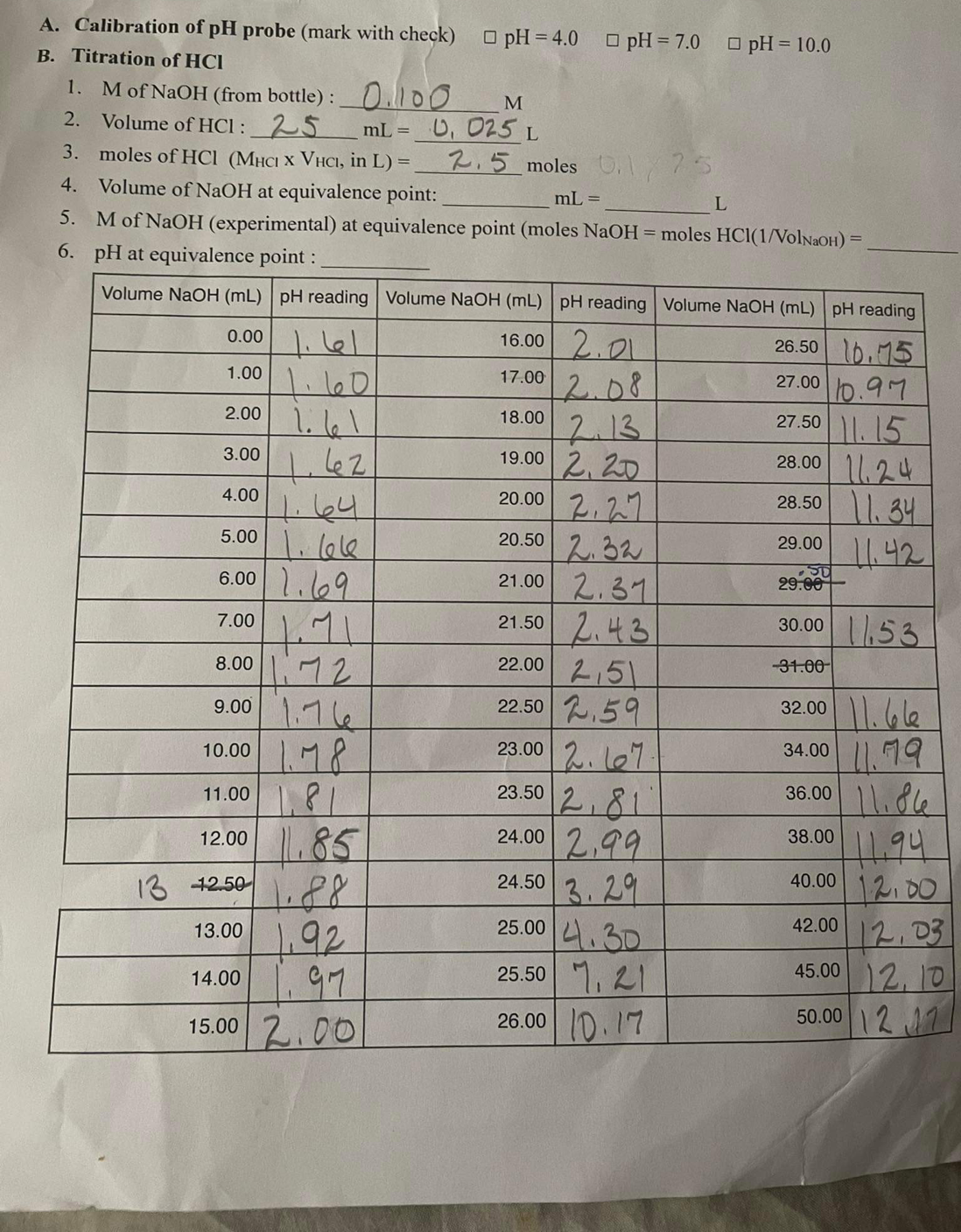
Transcribed Image Text:A. Calibration of pH probe (mark with check)
B. Titration of HCI
1. M of NaOH (from bottle):
2. Volume of HCI: 25
0.100
pH = 4.0 D pH = 7.0 E pH=10.0
M
mL = 0, 025 L
==
3. moles of HCI (MHCI X VHCI, in L) =
4. Volume of NaOH at equivalence point:
2.5 moles 0.1/75
mL =
L
5. M of NaOH (experimental) at equivalence point (moles NaOH = moles HCl(1/VolNaOH) =
6. pH at equivalence point:
Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading Volume NaOH (mL) pH reading
0.00 l
1.00
1.60
2.00
اما ١٠
16.00 2.0
26.50 10.15
17.00
2.08
27.00 0.97
18.00
2.13
27.50 11.15
3.00
42
19.00 2,20
28.00 11.24
4.00
20.00
1.64
2.27
28.50 1.34
5.00
20.50
2.32
29.00 11.42
6.00 1.69
21.00 2.31
29.00
7.00
8.00
1.71
11:72
21.50 2.43
30.00 11.53
22.00
31.00
2.51
9.00 1.76
22.50 2.59
32.00 11.66
10.00
1.78
23.00 2.7
34.00
11.79
11.00
81
23.50
2.81
36.00 11.86
12.00
11.85
24.00 2.99
38.00
11.94
13 42.50
1.88
24.50 3.29
40.00
12.00
13.00
1.92
25.00 4.30
42.00
12.03
14.00
1.97
25.50 7,21
45.00 12,10
15.00 2.00
26.00 0.17
50.00 127
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps

Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole