Content/24SSEM_CHEM_114 X Aktiv Chemistry - Work b Home | bartleby x + ← → с app.aktiv.com Question 8 of 39 Consider these electron transitions in a hydrogen atom: I. n 2 n 1 Il. n 3 n = 1 III. n=1 → n = 4 Which of the electron transitions would release the most energy? A B с | III D I, II, and III release the same amount of energy + Submit
Content/24SSEM_CHEM_114 X Aktiv Chemistry - Work b Home | bartleby x + ← → с app.aktiv.com Question 8 of 39 Consider these electron transitions in a hydrogen atom: I. n 2 n 1 Il. n 3 n = 1 III. n=1 → n = 4 Which of the electron transitions would release the most energy? A B с | III D I, II, and III release the same amount of energy + Submit
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter7: Electronic Structure
Section: Chapter Questions
Problem 7.95QE
Related questions
Question
Transcribed Image Text:Content/24SSEM_CHEM_114 X
Aktiv Chemistry - Work
b Home | bartleby
x +
← →
с
app.aktiv.com
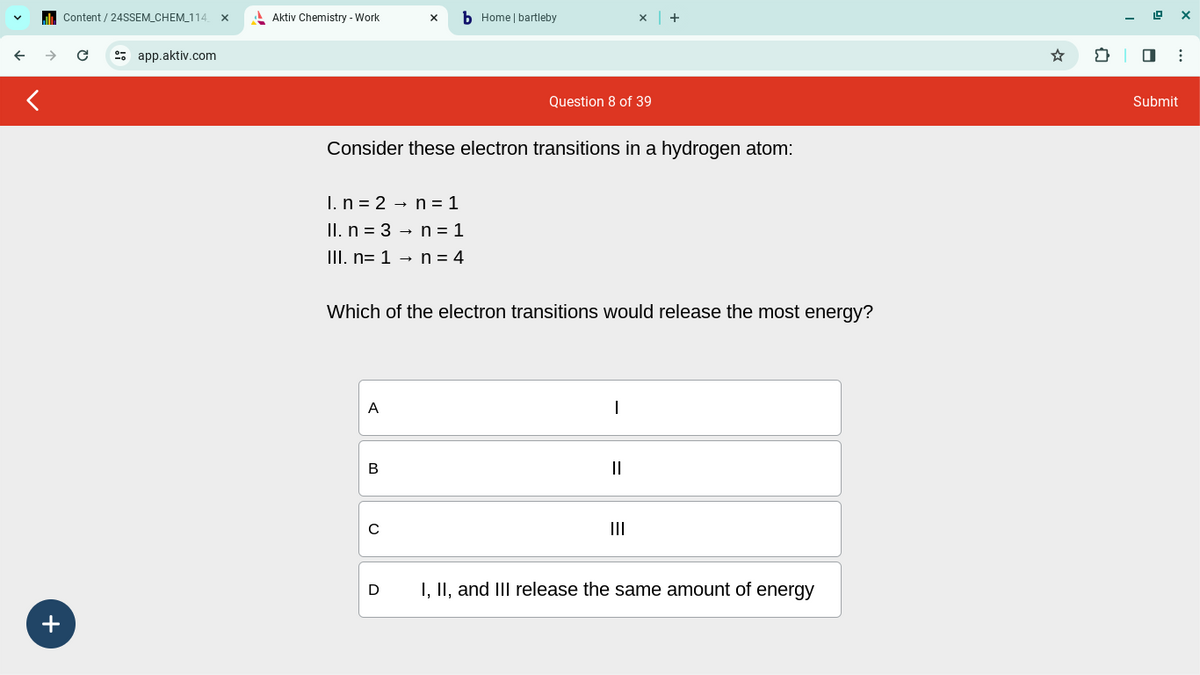
Question 8 of 39
Consider these electron transitions in a hydrogen atom:
I. n
2
n
1
Il. n 3
n = 1
III. n=1 →
n = 4
Which of the electron transitions would release the most energy?
A
B
с
|
III
D
I, II, and III release the same amount of energy
+
Submit
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning