Q: The production of ammonia is being studied. If the chemical reaction produced 0.0563 M ammonia after...
A: The rate of the formation of the product or the rate of disappearance of the reactants is known as t...
Q: How many seconds are there in a solar year (365.24 days )?
A: 1 day= 24h 1h= 60 min 1 min= 60 sec
Q: 1. CH;CH20- Na* 2. H*, CH;CH2OH он H. I ll. ...
A: We have to tell the specific information about product.
Q: What is the molality of a solution containing 6.85 g of urea [(NH2),CO] in 177.5 g of water?
A: Molar mass of urea = 60 gm
Q: Twenty-five mL of 0.100 M lactic acid (K, = 1.4 x 10-) is titrated with 0.097 M KOH. a) What is the ...
A:
Q: 2. A 230 ml ethyl alcohol was placed in a Pyrex glass and makes it full at 22°C. How much alcohol wi...
A: Given: Initial volume of ethyl alcohol = 230ml T1 = 22°C T2 = 100°C ΔT = (100-22)°C = 78°C
Q: 2. The full structural formulae of three organic compounds, P, Q and R, are shown below. H H H H HH ...
A: Isomers are those which have same molecular formula but different arrangement of atoms and molecules...
Q: For oxygen-18 with a charge of 1, the number of protons ,neutron ,and electrons
A: since you have asked multiple question, as per our company guidelines we are supposed to answer the ...
Q: Properties of Liquids and IMF example andbrief discussion
A: Liquids can be defined as fluids which do not have fixed shape but volume. For example: Water, Oil, ...
Q: In the presence of excess thiocyanate ion, SCN , the following reaction is first order in iron(III) ...
A: For a first order reaction., Rate constant = 1.27 second-1 And the reaction complete 87.9%.It means ...
Q: Give a definition of what polymers are, and what is their application or use in everyday life
A: Answer of the question given below,
Q: 4. The radioactive element 217At is a member of the e. series ... a. 4n b. 4n+1 c. 4n +2 d. 4n +3 e....
A: Heavy atomic nuclei are unstable and decay through emitting a sequence of alpha and beta particles. ...
Q: Complete and balance the following equation: Perchloric acid and lithium carbonate Add all of the ...
A: The solution is given below -
Q: What is the initial mass of copper (in g) in the Cu(NO3)2 solution. Cu(NO3)2(aq)+ 2NaOH(aq)-> 2NaNO3...
A: Ans. 63.5 g of Cu is present in Cu(NO3)2 solution
Q: A. Identify the name of the functional group where the given organic compounds belong (alcohol, ethe...
A: Functional groups are responsible for the characteristic reaction of compounds
Q: 2. A sample of material contains for its active components NaOH, NazCO3, NaHCO3, or compatible mixtu...
A:
Q: [H30*] (M) [ОН] (М) pOH pH Acidic or Basic а. 0.0059 b. 12.00
A:
Q: Explain the photosynthesis prcoess and respiration process in a tree.
A: Photosynthesis is a process by which plant turns water, sunlight and Corbon dioxide into oxygen and ...
Q: A solution prepared from 96 g of non-volatile non dissociating solute in 5.25 mole of toluene has a ...
A: Given data is as follows: The mass of the non-volatile non- dissociating solute = 96 g The moles of ...
Q: A fermenter was filled with 10L of 0.6 mol/L sodium sulfite solution containing 0.003M Cu2+ ion and ...
A: A numerical problem based on mole concept that is to be accomplished.
Q: The compound Pb(NO2)2 is an ionic compound. What are the ions of which it is composed? Cation formul...
A: The given compound is Pb(NO2)2. The ions in the given compound are Pb2+ and 2NO2-.
Q: For the following reaction , the rate constant at 373 K i s 0.69856 min-1 . 1. Find the order of th...
A: Introduction: The order of the reaction can be determined from the unit of the rate constant. We hav...
Q: Directions: Predict whether the entropy change of the system is each of the Sullowing is ACTIVITY 1 ...
A: The answer to the following question is-
Q: Solve for number 4 and 5.
A: The question is based upon intermolecular forces. The Intermolecular forces are the force which medi...
Q: Acetic acid and ethanol react to form ethyl acetate and water, like this: HCH,CO,(aq)+C,H,OH(aq) C,H...
A: Answer: This question is based on the Le-chatalier's principle, according to which, on changing any ...
Q: Major Classes of Molecule Functional Example Ionic or How will the functional g affect the m Group P...
A: Here we have to determine functional group , class of compound , example of compounds ,whether polar...
Q: 3. For Dan, use sketches of the orbitals to show that d., have B2g symmetry i.e. show how the orbita...
A:
Q: DISCUSS THE FOLLOWING DIPOLE DIPOLE FORCES,ION-DIPOLE FORCES, DISPERSION FORCES, THE HYDROGEN BOND
A: Since you have posted multiple questions, we are entitled to answer the first ony.
Q: (Use the lowest poscibie coefficients for all reactions.) Al + Fes O4 Al, 03 +Fe Al + Fes O4 Al2O3 +...
A: Al + Fe3O4 --------> Al2O3 + Fe Al 1 Al 2 Fe 3 Fe 1 O 4 O 3
Q: How much solute (lauric acid) do you need to add to the solvent wax (stearic acid) to make a 100g ca...
A: Well, In order to calculate the amount of lauric acid and solvent( stearic acid) we need to calculat...
Q: The activation energy (E,) for a certain biological reaction is 62.1 kJ/mol. If the rate constant (k...
A:
Q: Question #1 Determine the requested quantities for the following questions. Report your answer t 3 d...
A: The given speciation diagram is:
Q: Sodium metal requires a photon with a minimum energy of 4.41 x10-19 J to emit electrons. a. What is ...
A: The minimum energy to emit an electron from sodium metal is = 4.41×10-19 J The minimum frequency to ...
Q: 21. Propane (C3H8) reacts with oxygen gas to form carbon dioxide and water. How many grams of oxygen...
A: The balanced chemical equation, C3H8 + 5O2 ==> 3CO2 + 4H2O Given, 3.8 moles of propane.
Q: What is the name of the compound with the formula BBra ? What is the name of the compound with the f...
A:
Q: Henderson-Hasselbach equation b) Calculate the pH of a buffer solution that contains 0.0416 M sodiu...
A:
Q: The reaction of peroxydisulfate ion ( S,03-) with iodide ion (I) is S,0g (ag) + 31 (ag) → 2s0,7 (aq)...
A: For a reaction aA + bB →cC + dD rate = K [A]a [B]b
Q: How many grams of copper that consists of 1.54 x 1024 atoms of copper?
A: To convert atoms to grams, Step I - Convert atoms to moles 1 mol = 6.022 × 1023...
Q: Consider the stoichiometry of the reaction provided in the question(s) below. a. A laboratory stude...
A: A) Given mass of benzaldehyde=0.0812 g Molar mass of benzaldehyde = 106.12 g/mol Moles of reactant A...
Q: From the following formula, calculate the quantity of each ingredient required to make 3 lb (AV) of ...
A:
Q: PART A. Molecular Models octe Draw Lewis structures for each of the molecules listed below. Show non...
A: Here we have to write Lewis structure, molecular geometry, hybridisation and Lewis structure of all ...
Q: Discuss whether H-H bond should have IR activity in spectrum. If, what is the approximate wavelength...
A: We have to discuss whether the H-H bond should have IR (infrared) active or not. Hydrogen (H-H) is a...
Q: Consider the following hypothetical double displacement reaction: A3B (aq) + CD2 (aq) --> What is th...
A: In balanced reaction number of each atom on reactant side is equal to number of each atom on product...
Q: The 'H NMR of allyl bromide is given. Assign the signals to different protons. In spin decoupling ex...
A:
Q: 1. Using arrow formalism, show how the McLafferty rearrangement might occur in PHCOCH2CH2CH3. What v...
A: McLafferty rearrange is a characteristic fragmentation of carbonyl compound in mass spectroscopy. fo...
Q: what are the cell notations for the table 1?
A: While writing a cell notation we write anode or oxidation and in right side we write reduction react...
Q: ACTIVITY 2: Problem Solving on Standard Entropy 1. Determine S for the reaction: SO:(g) + H2O(1) –> ...
A:
Q: 8. Give IUPAC names for the following compounds: (a) CI „Br (b) CH3 (c) NH2 CH2CH2CHCH3 Br (d) CI CH...
A: IUPAC nomenclature is used for naming organic compounds. Full form of IUPAC is international union o...
Q: What is the name of the compound with the formula PbI,? What is the name of the compound with the fo...
A: Salt of any compound is form from cation and anions combinations and they are named accordingly
Q: ACTIVITY/EXERCISES Direction: Identify the given substances if it is polar or nonpolar, and which ty...
A:


Reaction rate:
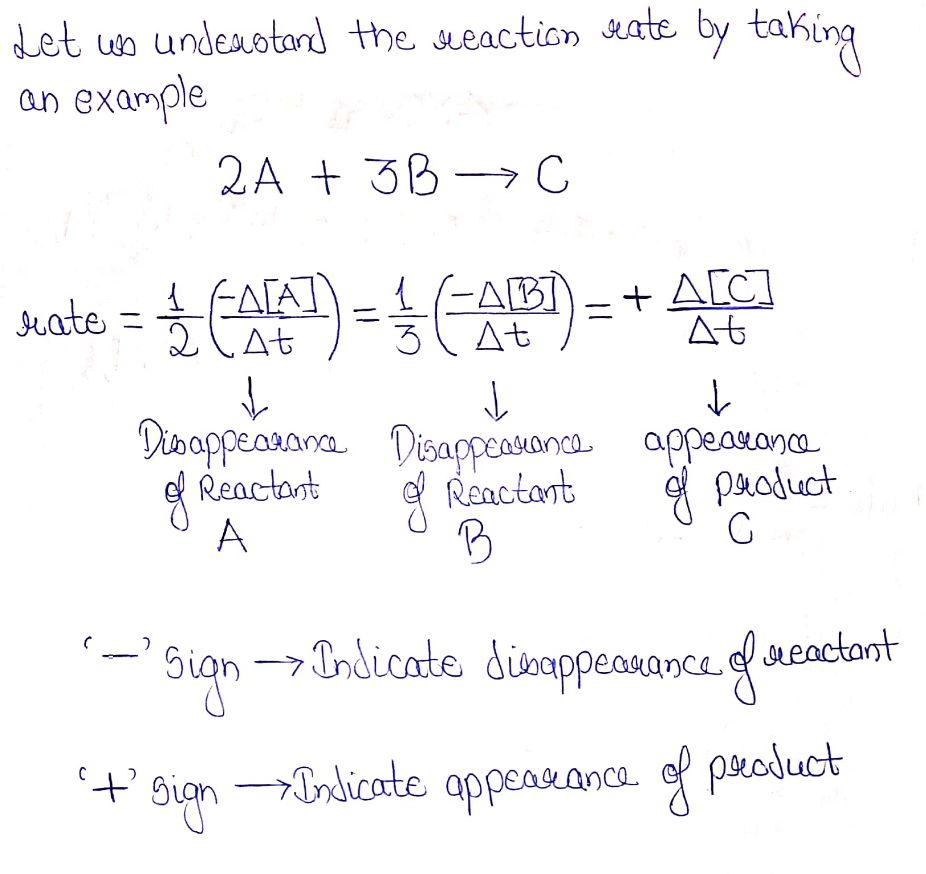
Step by step
Solved in 3 steps with 2 images

- 9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in water: NH4NO3(s)NH4+(aq)+NO3(aq) H= 25.69 kJ A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25J g-l K-l, and that the heat capacity of the bag itself is small enough to be neglected.)When a mixture of hydrogen and bromine is maintained at normal atmospheric pressure and heated above 200. °C in a closed container, the hydrogen and bromine react to form hydrogen bromide and a gas-phase equilibrium is established. Write a balanced chemical equation for the equilibrium reaction. Use bond enthalpies from Table 6.2 ( Sec. 6-6b) to estimate the enthalpy change for the reaction. Based on your answers to parts (a) and (b), which is more important in determining the position of this equilibrium, the entropy effect or the energy effect? In which direction will the equilibrium shift as the temperature increases above 200. °C? Explain. Suppose that the pressure were increased to triple its initial value. In which direction would the equilibrium shift? Why is the equilibrium not established at room temperature?Reword the statement in Question 109 so that it is always true. Criticize this statement: Provided it occurs at an appreciable rate, any chemical reaction for which rG 0 will proceed until all reactants have been converted toproducts.
- The equilibrium constant for a certain reaction increases by a factor of 6.67 when the temperature is increased from 300.0 K to 350.0 K. Calculate the standard change in enthalpy (H) for this reaction (assuming H is temperature-independent).5.11. Determine the numerical value of Q for the reaction conditions indicated.Calculate H when a 38-g sample of glucose, C6H12O6(s), burns in excess O2(g) to form CO2(g) and H2O() in a reaction at constant pressure and 298.15 K.
- The following reaction occurs in pure water: H2O(l)+H2O(l)H3O+(aq)+OH-(aq) which is often abbreviated as H2O(l)H+(aq)+OH-(aq) For this reaction, G = 79.9 kJ/mol at 25C. Calculate the value of G for this reaction at 25C when [OH] = 0.15 M and [H+] = 0.71 M.Determine rxnH 25 C for the following reaction: NO g O2 g NO2 g This reaction is a major participant in the formation of smog.When 7.11 g NH4NO3 is added to 100 mL water, the temperature of the calorimeter contents decreases from 22.1 C to 17.1 C. Assuming that the mixture has the same specific heat as water and a mass of 107 g, calculate the heat q. Is the dissolution of ammonium nitrate exothermic or endothermic?
- Adenosine triphosphate, ATP, is used as a free-energy source by biological cells. (See the essay on page 624.) ATP hydrolyzes in the presence of enzymes to give ADP: ATP(aq)+H2O(l)ADP(aq)+H2PO4(aq);G=30.5kJ/molat25C Consider a hypothetical biochemical reaction of molecule A to give molecule B: A(aq)B(aq);G=+15.0kJ/molat25C Calculate the ratio [B]/[A] at 25C at equilibrium. Now consider this reaction coupled to the reaction for the hydrolysis of ATP: A(aq)+ATP(aq)+H2O(l)B(aq)+ADP(aq)+H2PO4(aq) If a cell maintains a high ratio of ATP to ADP and H2PO4 by continuously making ATP, the conversion of A to B can be made highly spontaneous. A characteristic value of this ratio is [ATP][ADP][H2PO4]=500 Calculate the ratio [B][A] in this case and compare it with the uncoupled reaction. Compared with the uncoupled reaction, how much larger is this ratio when coupled to the hydrolysis of ATP?12.108 A nuclear engineer is considering the effect of discharging waste heat from a power plant into a lake and estimates that this may warm the water locally to 25 °C. One question to be considered is the effect of this temperature change on the uptake of CO2 by the water. The equilibrium constant for the reaction CO2+H2OH2CO3 ; is K=1.7103 at 25 °C. Because bonds form, the reaction is exothermic. (a) Will this reaction progress further toward products at higher temperatures near the water discharge with its warmer water than it would in the cooler lake water? Explain your reasoning. (b) Carbonic acid has a Kaof 2.5104 at 25 °C. What is the equilibrium constant for the CO2+2H2OHCO3+H3O+? (c) What additional factor should the engineer be considering about CO2 gas, probably before considering this reaction chemistry?











