In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50 °C NH4NCO(aq)(NH2)½CO(aq) the following data were obtained: NH,NCO], M 0.715 8.95x10-2 1.05x103 0.358 0.179 minutes 150 449 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.715 M is | min and when the starting concentration is 0.358 M is min. (2) The average A(1/[NH,NCO]) / At from t = 0 min to t = 150 min i [ The average A(1/[NH4NCO]) / At from t= 150 min to t = 449 min is | M' min. м' min'. (3) Based on these data, the rate constant for this | order reaction is м' min'.
In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50 °C NH4NCO(aq)(NH2)½CO(aq) the following data were obtained: NH,NCO], M 0.715 8.95x10-2 1.05x103 0.358 0.179 minutes 150 449 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.715 M is | min and when the starting concentration is 0.358 M is min. (2) The average A(1/[NH,NCO]) / At from t = 0 min to t = 150 min i [ The average A(1/[NH4NCO]) / At from t= 150 min to t = 449 min is | M' min. м' min'. (3) Based on these data, the rate constant for this | order reaction is м' min'.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
![In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50 °C
NH,NCO(aq)→(NH2)½CO(aq)
the following data were obtained:
[NH,NCO], M |0.715
8.95x10-2
1.05x103
0.358
0.179
minutes
150
449
Hint: It is not necessary to graph these data.
(1)
The observed half life for this reaction when the starting concentration is 0.715 M is
min and when the starting concentration is 0.358 M
is
min.
(2)
The average A(1/[NHẠNCO]) / At from t= 0 min to t= 150 min i |
The average A(1/[NH,NCO]) / At from t= 150 min to t= 449 min is
M min.
M' min!.
(3)
Based on these data, the rate constant for this
| order reaction is
M' min!.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fdce0254f-c57a-4702-9013-ef864d8f78cf%2Ffa80fe91-81bb-477d-ac64-e81683ebfe92%2Fyxtcuxa_processed.png&w=3840&q=75)
Transcribed Image Text:In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50 °C
NH,NCO(aq)→(NH2)½CO(aq)
the following data were obtained:
[NH,NCO], M |0.715
8.95x10-2
1.05x103
0.358
0.179
minutes
150
449
Hint: It is not necessary to graph these data.
(1)
The observed half life for this reaction when the starting concentration is 0.715 M is
min and when the starting concentration is 0.358 M
is
min.
(2)
The average A(1/[NHẠNCO]) / At from t= 0 min to t= 150 min i |
The average A(1/[NH,NCO]) / At from t= 150 min to t= 449 min is
M min.
M' min!.
(3)
Based on these data, the rate constant for this
| order reaction is
M' min!.
Expert Solution
Step 1
The reaction given is,
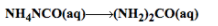
And the kinetics data given is,

Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning