m2) The interatomic interaction energy for two atoms (with masses m2) of a diatomic molecule is given as: Eint. (r) = B r12 r A where is the distance between these two atoms (A and B are positive con- ndstants). Find the binding energy, equilibrium (bond) distance between two teratoms and vibrational frequency of the molecule in terms of given parameters.
m2) The interatomic interaction energy for two atoms (with masses m2) of a diatomic molecule is given as: Eint. (r) = B r12 r A where is the distance between these two atoms (A and B are positive con- ndstants). Find the binding energy, equilibrium (bond) distance between two teratoms and vibrational frequency of the molecule in terms of given parameters.
Related questions
Question
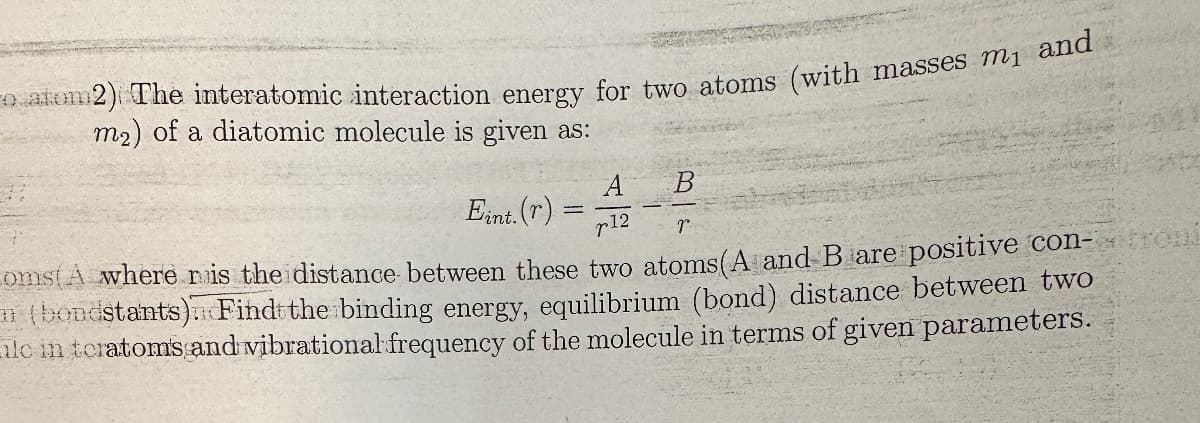
Transcribed Image Text:o atom2) The interatomic interaction energy for two atoms (with masses m₁ and
m2) of a diatomic molecule is given as:
A
B
Eint. (r)
12
r
oms(A where r is the distance between these two atoms (A and B are positive con-troni
(bondstants) Find the binding energy, equilibrium (bond) distance between two
ilc in teratoms and vibrational frequency of the molecule in terms of given parameters.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images
