Problem 6: A rigid tank of volume V = 0.015 m³ contains carbon monoxide at a temperature of To = 21° C and a pressure of Po = 9.00 × 105 Pa. This molecule should be treated as a diatomic ideal gas with active vibrational modes. Part (a) In this model, how many degrees of freedom does each molecule of carbon monoxide have? 7 ✓ Correct! Part (b) The temperature of the gas increases by 10° C. Select the process that has occurred from the choices below. This is an isochoric process. ✓ Correct! Part (c) Calculate the pressure of the gas in pascal at this increased temperature. P=9.3*105 P = 9.300 × 105 ✓ Correct! Part (d) Calculate the change to the internal energy of the gas in joules. AU = 290.51 AS = 66.90 Part (e) Calculate the change in the entropy of the gas in joules per kelvin. AS = 66.90 * Attempts Remain
Problem 6: A rigid tank of volume V = 0.015 m³ contains carbon monoxide at a temperature of To = 21° C and a pressure of Po = 9.00 × 105 Pa. This molecule should be treated as a diatomic ideal gas with active vibrational modes. Part (a) In this model, how many degrees of freedom does each molecule of carbon monoxide have? 7 ✓ Correct! Part (b) The temperature of the gas increases by 10° C. Select the process that has occurred from the choices below. This is an isochoric process. ✓ Correct! Part (c) Calculate the pressure of the gas in pascal at this increased temperature. P=9.3*105 P = 9.300 × 105 ✓ Correct! Part (d) Calculate the change to the internal energy of the gas in joules. AU = 290.51 AS = 66.90 Part (e) Calculate the change in the entropy of the gas in joules per kelvin. AS = 66.90 * Attempts Remain
Related questions
Question
Plz correct solution part d and e.
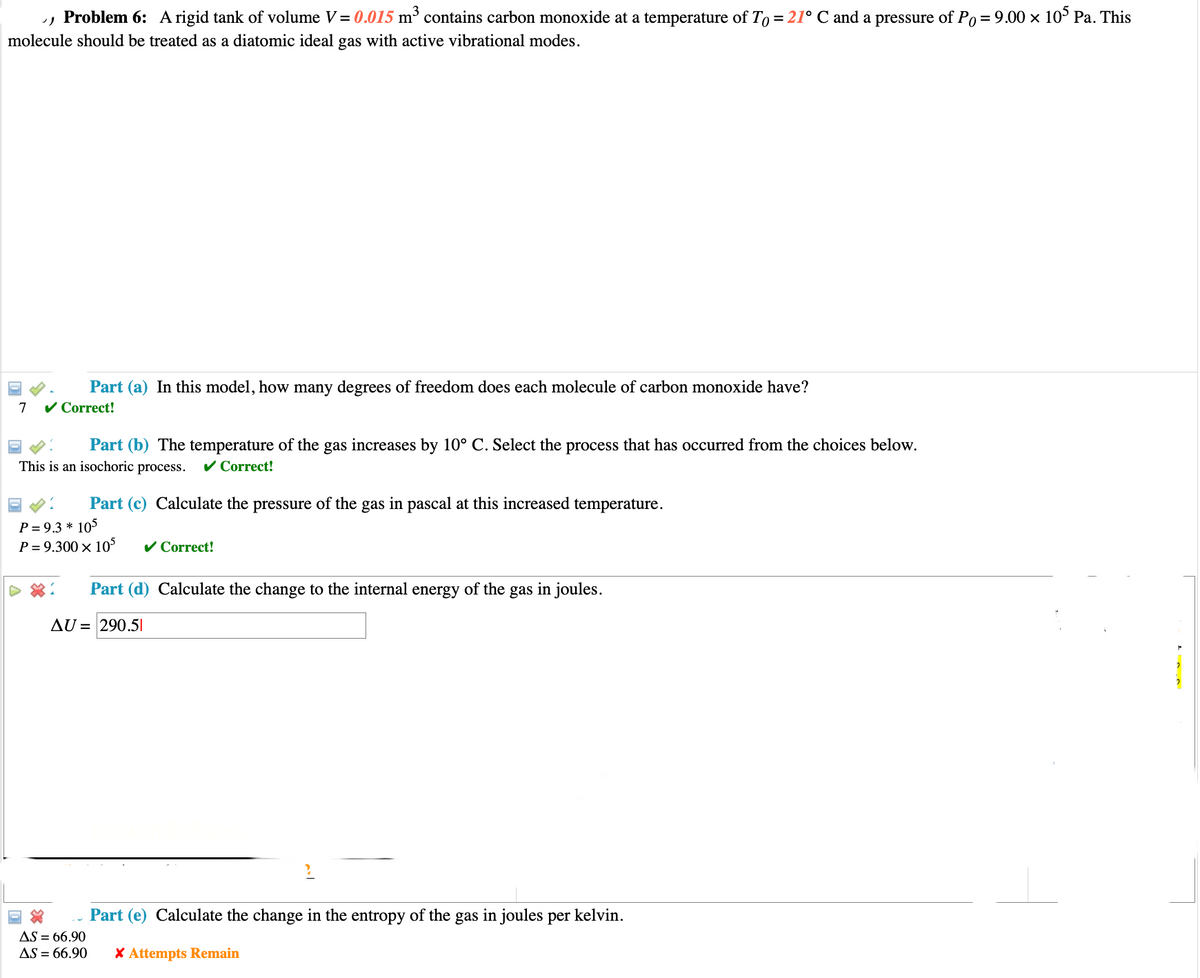
Transcribed Image Text:› Problem 6: A rigid tank of volume V = 0.015 m³ contains carbon monoxide at a temperature of To = 21° C and a pressure of Po = 9.00 × 105 Pa. This
molecule should be treated as a diatomic ideal gas with active vibrational modes.
7
Part (a) In this model, how many degrees of freedom does each molecule of carbon monoxide have?
✓ Correct!
Part (b) The temperature of the gas increases by 10° C. Select the process that has occurred from the choices below.
This is an isochoric process. ✓ Correct!
Part (c) Calculate the pressure of the gas in pascal at this increased temperature.
P = 9.3 * 105
P = 9.300 × 105
✓ Correct!
Part (d) Calculate the change to the internal energy of the gas in joules.
AU = 290.5|
AS = 66.90
Part (e) Calculate the change in the entropy of the gas in joules per kelvin.
AS = 66.90 * Attempts Remain
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images
