Q4/ The ideal gas equation of states is given by: PV = nRT Where: P is the pressure, V is the volume, T is the temperature, R=0.08206 (L atm)/(mol K) is the ideal gas constant, and n is the number of moles. Real gases, especially at high pressures, deviate from this behavior. Their responses can be modeled with the van der Waals equation: nRT using matlab V-nb P n² a + = 0 v2= Where a and b are gas constants. For Cl₂ a = 6.579 L'atm/mol², and b = 0.0562 L/mol. (a) Write a code which asks the user to insert n, T, a, b and then plots P versus V on one figure - two plots for both equations if the volume range is (0.5
Q4/ The ideal gas equation of states is given by: PV = nRT Where: P is the pressure, V is the volume, T is the temperature, R=0.08206 (L atm)/(mol K) is the ideal gas constant, and n is the number of moles. Real gases, especially at high pressures, deviate from this behavior. Their responses can be modeled with the van der Waals equation: nRT using matlab V-nb P n² a + = 0 v2= Where a and b are gas constants. For Cl₂ a = 6.579 L'atm/mol², and b = 0.0562 L/mol. (a) Write a code which asks the user to insert n, T, a, b and then plots P versus V on one figure - two plots for both equations if the volume range is (0.5
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
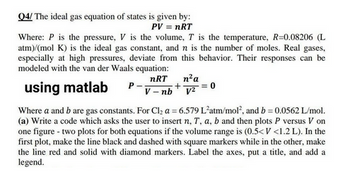
Transcribed Image Text:Q4/ The ideal gas equation of states is given by:
PV = nRT
Where: P is the pressure, V is the volume, T is the temperature, R=0.08206 (L
atm)/(mol K) is the ideal gas constant, and n is the number of moles. Real gases,
especially at high pressures, deviate from this behavior. Their responses can be
modeled with the van der Waals equation:
nRT
using matlab
V-nb
P
n² a
+ = 0
v2=
Where a and b are gas constants. For Cl₂ a = 6.579 L'atm/mol², and b = 0.0562 L/mol.
(a) Write a code which asks the user to insert n, T, a, b and then plots P versus V on
one figure - two plots for both equations if the volume range is (0.5<V <1.2 L). In the
first plot, make the line black and dashed with square markers while in the other, make
the line red and solid with diamond markers. Label the axes, put a title, and add a
legend.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, computer-science and related others by exploring similar questions and additional content below.Recommended textbooks for you

C++ for Engineers and Scientists
Computer Science
ISBN:
9781133187844
Author:
Bronson, Gary J.
Publisher:
Course Technology Ptr

C++ for Engineers and Scientists
Computer Science
ISBN:
9781133187844
Author:
Bronson, Gary J.
Publisher:
Course Technology Ptr
