
Part a:
Number of moles of HCl solution = 0.015 M × 0.05 L = 0.00075 mol
Number of moles of NaOH solution = 0.015 M × 0.01 L = 0.00015 mol
0.00015 mol of NaOH neutralizes 0.00015 mol of HCl and forms 0.00015 mol of NaCl. The remaining number of moles of HCl is equal to 0.00075 – 0.00015 mol = 0.0006 mol. The pH of the solution is obtained due to 0.0006 mol of HCl since NaCl is a salt of strong acid and strong base and such salts do not undergo hydrolysis. The neutralization reaction between NaOH and HCl can be shown by the following balanced chemical equation:
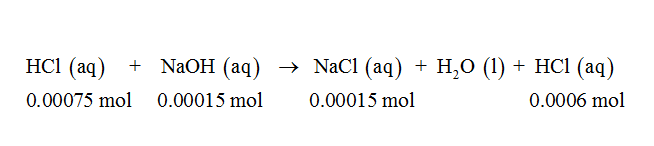
Since HCl is a strong acid, it completely dissociates into hydrogen ions and chloride ions. The total number of moles of hydrogen ions released from 0.0006 mol of HCl is equal to 0.0006 mol and the total volume of the solution is equal to 0.05 L + 0.01 L = 0.06 L. The pH of the solution can be calculated as follows-
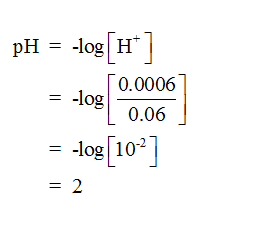
Step by step
Solved in 4 steps with 4 images









