Table 3-pH Meter Measurement Values of Ammonia/Ammonium Buffer Part C- Buffers pH of concentrated buffer pH of diluted buffer pH of buffer + HCL pH of buffer + NaOH Table 4-pH Meter Measurement Value of Non-Buffered Solutions Part D - Comparison to water pH of pure water 9.60 9.36 9.32 9.45 pH of water + HCI pH of water + NaOH 10.02 5.42 6.29 Page 2 of 8 6) In both Part C and Part D five drops of acid and five drops of base were added to around 30 mL of buffer solution and water respectively. a) Comment and discuss the difference between the pH change in the buffered and non-buffered solutions. Explanation of how that correlates to pH data Put your answer here in this box! b) What happened to the protons of the acid when it was dripped into the pure water? Page 6 of 8 Explanation of how that correlates to pH data Put your answer here in this box! c) What happened to the protons of the acid when it was dripped into the buffered solution? Explanation of how that correlates to pH data Put your answer here in this box!
Table 3-pH Meter Measurement Values of Ammonia/Ammonium Buffer Part C- Buffers pH of concentrated buffer pH of diluted buffer pH of buffer + HCL pH of buffer + NaOH Table 4-pH Meter Measurement Value of Non-Buffered Solutions Part D - Comparison to water pH of pure water 9.60 9.36 9.32 9.45 pH of water + HCI pH of water + NaOH 10.02 5.42 6.29 Page 2 of 8 6) In both Part C and Part D five drops of acid and five drops of base were added to around 30 mL of buffer solution and water respectively. a) Comment and discuss the difference between the pH change in the buffered and non-buffered solutions. Explanation of how that correlates to pH data Put your answer here in this box! b) What happened to the protons of the acid when it was dripped into the pure water? Page 6 of 8 Explanation of how that correlates to pH data Put your answer here in this box! c) What happened to the protons of the acid when it was dripped into the buffered solution? Explanation of how that correlates to pH data Put your answer here in this box!
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
I need help with question 6?
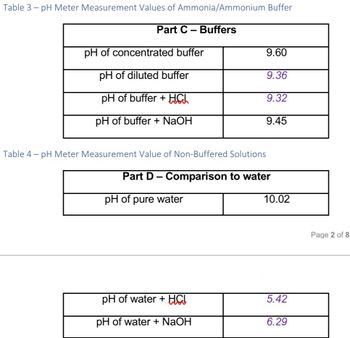
Transcribed Image Text:Table 3-pH Meter Measurement Values of Ammonia/Ammonium Buffer
Part C- Buffers
pH of concentrated buffer
pH of diluted buffer
pH of buffer + HCL
pH of buffer + NaOH
Table 4-pH Meter Measurement Value of Non-Buffered Solutions
Part D - Comparison to water
pH of pure water
9.60
9.36
9.32
9.45
pH of water + HCI
pH of water + NaOH
10.02
5.42
6.29
Page 2 of 8

Transcribed Image Text:6) In both Part C and Part D five drops of acid and five drops of base were added
to around 30 mL of buffer solution and water respectively.
a) Comment and discuss the difference between the pH change in the
buffered and non-buffered solutions.
Explanation of how that correlates to pH data
Put your answer here in this box!
b) What happened to the protons of the acid when it was dripped into the
pure water?
Page 6 of 8
Explanation of how that correlates to pH data
Put your answer here in this box!
c) What happened to the protons of the acid when it was dripped into the
buffered solution?
Explanation of how that correlates to pH data
Put your answer here in this box!
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
