The following diagram is a shell representation of an atom. When the atom is exposed to light, the electron absorbs light and undergoes excitation from ground state to excited state as shown in the diagram. ground state excited stete a) What are the names of the valence shells in both ground and excited states as per the diagram? b) Write the value of Principal Quantum number (n) both for ground and excited state. c) Write the value of Azimuthal Quantum number (I) both for ground and excited state. d) Write a possible Isotope for this atom in the proper symbolism.
The following diagram is a shell representation of an atom. When the atom is exposed to light, the electron absorbs light and undergoes excitation from ground state to excited state as shown in the diagram. ground state excited stete a) What are the names of the valence shells in both ground and excited states as per the diagram? b) Write the value of Principal Quantum number (n) both for ground and excited state. c) Write the value of Azimuthal Quantum number (I) both for ground and excited state. d) Write a possible Isotope for this atom in the proper symbolism.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 45AP: Suppose an atom in an excited state can return to the ground state in two steps. It first falls to...
Related questions
Question
100%
4- Please I want answer sub-parts (c) and (d) with steps and clear typing or hand writing. Many thanks
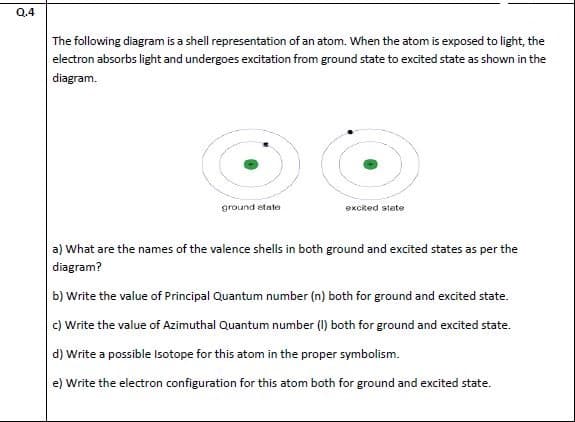
Transcribed Image Text:The following diagram is a shell representation of an atom. When the atom is exposed to light, the
electron absorbs light and undergoes excitation from ground state to excited state as shown in the
diagram.
ground state
excited stete
a) What are the names of the valence shells in both ground and excited states as per the
diagram?
b) Write the value of Principal Quantum number (n) both for ground and excited state.
c) Write the value of Azimuthal Quantum number (I) both for ground and excited state.
d) Write a possible Isotope for this atom in the proper symbolism.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,