The following reaction below shows an example of an intramolecular acid-base reaction where the acid and base are on the same molecule. Complete the following statements relating to this reaction. H H. :0 :S :0⁹ 3.We would A. I B. <1 C. >1 0: 1. Equilibrium favors the formation of the A. Products, B. Products + Reactants at equal concentrations, C. reactant's 2.Major reason why one side of the reaction is favored is because_ Hybridization of charged atom, Electrane naged atom, Industrients) Expect Key of reaction to be. resonance decolication of charged alon, or Size of Charged atom 4. we would expect this rxn to be; A.endergonic B. exergonic
The following reaction below shows an example of an intramolecular acid-base reaction where the acid and base are on the same molecule. Complete the following statements relating to this reaction. H H. :0 :S :0⁹ 3.We would A. I B. <1 C. >1 0: 1. Equilibrium favors the formation of the A. Products, B. Products + Reactants at equal concentrations, C. reactant's 2.Major reason why one side of the reaction is favored is because_ Hybridization of charged atom, Electrane naged atom, Industrients) Expect Key of reaction to be. resonance decolication of charged alon, or Size of Charged atom 4. we would expect this rxn to be; A.endergonic B. exergonic
Related questions
Question
/&/&:&&;&;&;&;&
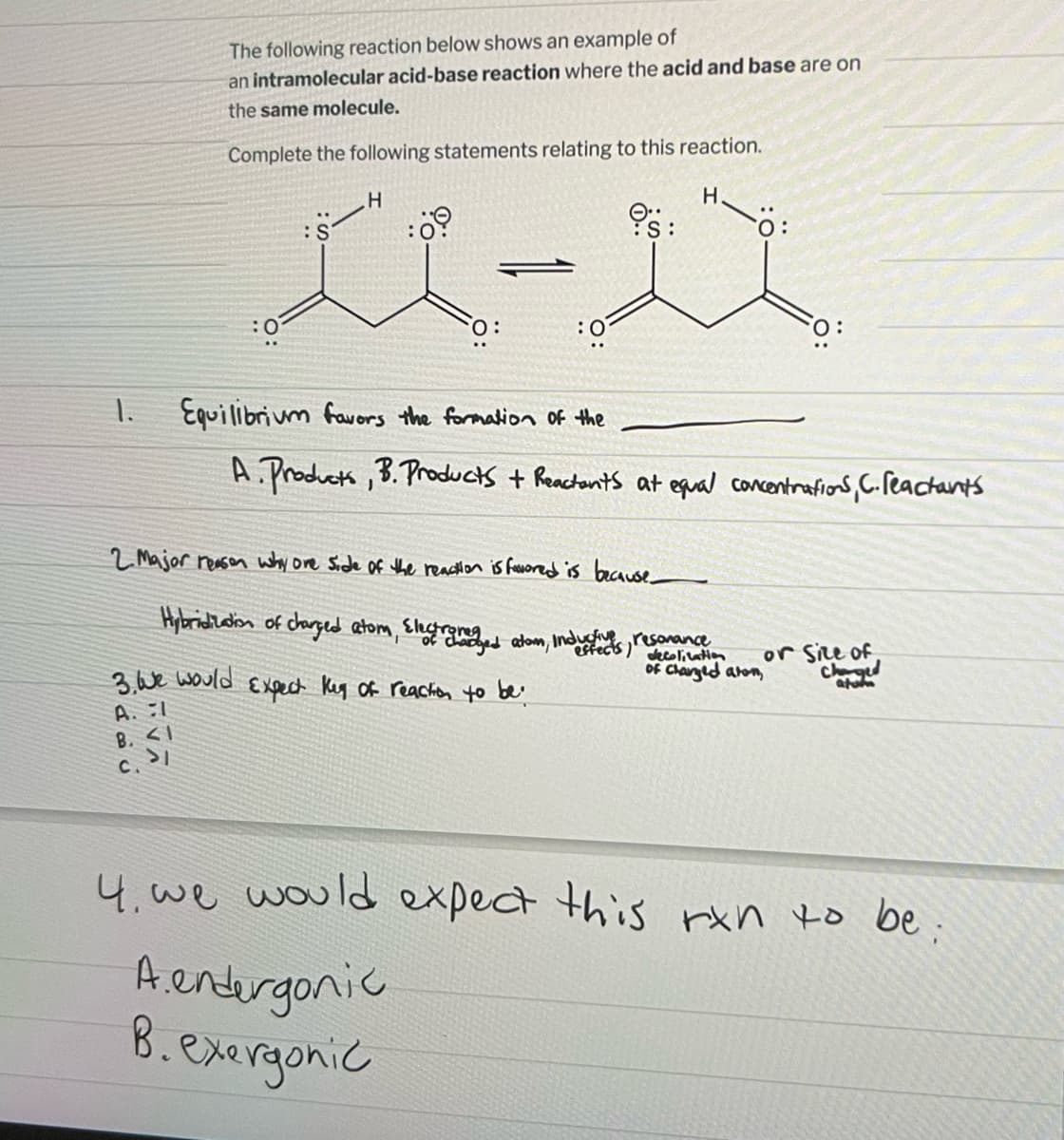
Transcribed Image Text:The following reaction below shows an example of
an intramolecular acid-base reaction where the acid and base are on
the same molecule.
Complete the following statements relating to this reaction.
H
H.
:0
:S
3. We would
A. I
B. <1
c. 31
:0⁹
0:
Equilibrium favors the formation of the
A. Products, B. Products + Reactants at equal concentrations, C. reactant's
2.Major reason why one side of the reaction is favored is because_
Hybridization of charged atom, Electromedaged atom, induced as
Expect Key of reaction to be
resonance
decolication
of charged alon,
or Size of
Charged
atom
4. we would expect this rxn to be;
A.endergonic
B. exergonic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps
