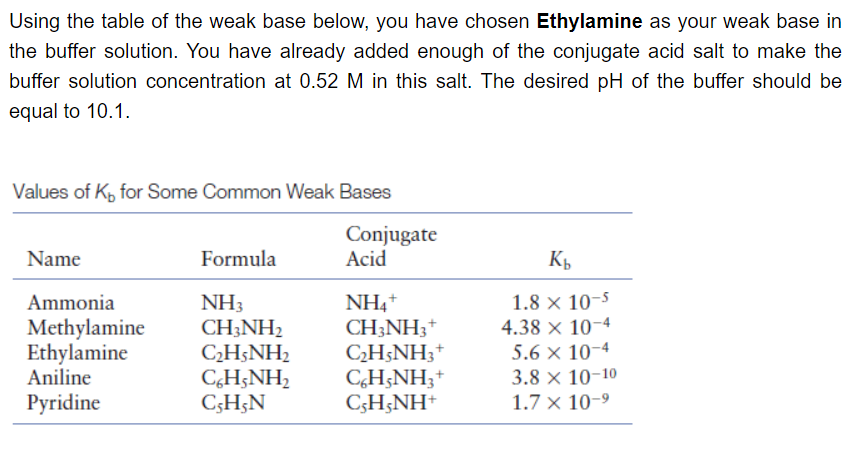
To prepare the buffer in the previous problem, a total volume of 500 mL is required. The stock solution of the weak base is available at 1 gallon with a concentration of 0.99 M. (Remember the lesson on dilution: M1V1=M2V2) 4. How many mL should be taken from the stock solution to prepare the desired buffer solution? (Write your answer in 3 decimal places without the unit). PREVIOUS PROBLEM:
To prepare the buffer in the previous problem, a total volume of 500 mL is required. The stock solution of the weak base is available at 1 gallon with a concentration of 0.99 M. (Remember the lesson on dilution: M1V1=M2V2)
4. How many mL should be taken from the stock solution to prepare the desired buffer solution? (Write your answer in 3 decimal places without the unit).
PREVIOUS PROBLEM:
Step by step
Solved in 3 steps with 3 images

to prepare the buffer in the previous problem, a total volume of 500 mL is required. The stock solution of the weak base is available at 1 gallon with a concentration of 0.45 M. (Remember the lesson on dilution: M1V1=M2V2)
4. How many mL should be taken from the stock solution to prepare the desired buffer solution? (Write your answer in 2 decimal places without the unit).








