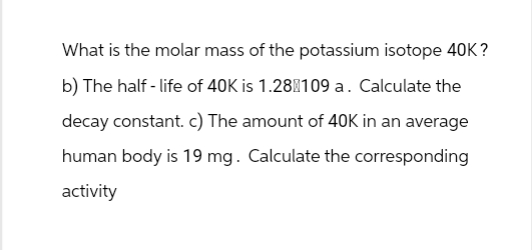
What is the molar mass of the potassium isotope 40k b) The half-life of 40K is 1.28 109 a. Calculate the decay constant. c) The amount of 40K in an average human body is 19 mg. Calculate the corresponding activity
Q: What is the path difference between two green (530 nm) rays that cause the darkest spot between 1st…
A: Given that the wavelength (λ) of the green light is 530 nm, the path difference (Δ) for the darkest…
Q: A 17.0-μF capacitor is charged by a 150.0-V power supply, then disconnected from the power and…
A: Solution Given data Capacitance C=17μF=17×10−6FPotential difference V=150VInductor…
Q: A -13.4nC point charge and a +23.1nC point charge are 18.0cm apart on the x-axis. What is the…
A: point charges areq1=-13.4nCq2=+23.1nCseparation betwee the chargesd=18cm
Q: 7. A ball is thrown vertically into the air at 40m/s. After 3.5 seconds, another ball is thrown…
A: Solution to Q. No. 7
Q: 5. A cylinder with infinite length, relative permittivity of ε,, and radius of R is charged with…
A:
Q: Hello, I am stuck on the practice problem. I'm not sure I am doing it right. Could you help guide me…
A:
Q: The half-life of radium is about 1600 years and its atomic number is 226. Find the percentage of…
A: After 100 years, the percentage of undecayed atoms left =95.76% The activity of 1mg of radium sample…
Q: Bonus 2 (2 pts) When angular momentum f = 3, draw all possible angular momentum distributions in 3D,…
A: The minimum radius is 2π3h. Please see the figure in explanation for details.Explanation:Step 1:…
Q: A particle has an initial speed vo. It makes a glancing collision with a second particle of equal…
A: According to conservation of linear momentum,momentum before collision=momentum after…
Q: The cosmic microwave background radiation is strong evidence for the Big Bang theory because it: (a)…
A: The objective of the question is to understand why the cosmic microwave background radiation (CMBR)…
Q: Hypothesis:If I give a hearing test to 5 boys and 5 girls . Then the girls will be able to hear…
A: Hypothesis:If I give a hearing test to 5 boys and 5 girls . Then the girls will be able to hear…
Q: a. b. Find the work done by the force of friction. Find the coefficient of friction.
A: The work energy theorem states that the work done on any object is equal to the difference between…
Q: he centre of mass in
A:
Q: The transfer function G(s) = X(s)/U(s).
A: The equivalent lumped mass of the system can be found using the formula:me=mc+ρALwhere ρ is the…
Q: A gas cell of length 50 mm is placed in one arm of a Michelson interferometer, with a mercury vapour…
A: In Michelson Interferometer, the number of fringes traversed is given by:
Q: 7. The oscillations in air pressure representing the sound wave for a musical tone can be modeled by…
A: Given Data:The equation of the sound wave is..To Draw:The complete cycle of the given sound wave.
Q: I need the to explain the given value : mx = 7.0164 (how he got it) Ep out and Ep in what does it…
A: mx = 7.0164The value mx=7.0164 likely represents the mass number of a specific isotope. For 7Li, the…
Q: 14) An electron is moving upward with speed 'v' in a magnetic field that points into the page. Which…
A:
Q: In the figure below, a long circular pipe with outside radius R = 3.2 cm carries a (uniformly…
A:
Q: An L-R-C series circuit has 0.300 H and C = 4.00 μF. Calculate the angular frequency of oscillation…
A: The objective of the question is to find the value of resistance that gives critical damping and the…
Q: 300 FIS) 101b Ms 0 15 MK=0.1 Find work done by friction (ft-lb) after the block is pushed 6ft 6ft F…
A:
Q: Metal object, with mass mm of 7 kg, temperature Tm of 355 K and specific heat capacity Cp,m of 490.0…
A:
Q: A perfectly flat piece of glass (n = 1.50) is placed over a perfectly flat piece of black plastic (n…
A: Given that : The refractive index of the glass n1=1.5 The refractive index of the glass n2=1.2 The…
Q: The mass density of Iron is 7.874 g/cm 3 ,a) What is the critical angle of total reflection from the…
A: The final answer is the same as the explanation underneath.Explanation:a. To find the critical angle…
Q: About how fast would you have to be driving toward a red (A = 685 nm) stoplight for it to appear…
A: Given Data:The wavelength of red light is λr=685 nm.The wavelength of red light is λr=532 nm.To…
Q: 2. A wave has the form y = А сos(2лx/λ+л/3) when x 0, the wavelength is 1/2. By applying con-…
A: For x<0 the wave is given asy=Acos(2πλ+π3)Let ϕ be the phase and A' be the amplitude of wave for…
Q: A transparent optical material has a refractive index of 1.8. What is the Fresnel Reflectivity…
A: A transparent optical material has a refractive index of 1.8. What is the Fresnel Reflectivity…
Q: A parallel plate capacitor is separated paraffined paper 0.001 cm thick (Ir The effective size of…
A:
Q: A worker pours 1.270 kg of molten lead at a temperature of 327.3 °C into 0.5080 kg of water at a…
A:
Q: F (kN) LANDING Consider the force versus time recording shown in Fig. 11.9 for an athlete making…
A: GivenConsider the force versus time recording shown in Fig. 11.9 for an athlete making vertical…
Q: Raising the temperature of a balloon at constant pressure requires a combination of heat energy Q…
A: Moles of helium n = 0.5 moles temperature to be raised at constant pressure = ( T2 - T1 ) = 15…
Q: a 65kg cyclist on a 15kg bicycle starts from rest and increases his speed to 12m/s in 20s a) what…
A: The objective of the question is to calculate the power output of the cyclist and the efficiency of…
Q: closed path on a rectangular prism, an
A: A net is a 2D representation of a 3D shape that can be folded to form the original 3D shape. In this…
Q: Binary Code
A: In the digital world, information flows as 0s and 1s. Standard binary uses these bits to represent…
Q: The physical length of the ruler as a fraction of the circumference of the big circle is the same as…
A: We have to find the conversion factor
Q: (a) Find the equivalent resistance of the circuit in Figure P18.8. (R15.00 S2, R2 = 11.0 (2) Ω (b)…
A: We will find the equivalent resistance of the circuit using expressions for series and parallel…
Q: ge e potential energy be
A:
Q: y radians of rotation c
A: We Know Angular Velocity, ω=vrWhere v is spider's speed r is distance from the axle of…
Q: Occasionally, huge icebergs are found floating on the ocean's currents. Suppose one such iceberg is…
A:
Q: ₁-mx=0 gm+2-my-0 x-sin(0)=0 y-l cos(0)=0 12 sin() cos(0) = 0 The first two equations are the…
A: According to Newton's second law of motion, force acting on a particle of mass m having acceleration…
Q: 4. A copper bar lies between two heat sources given by T₁ = 100°C and Tc = 20°C. The area of the…
A: We know that when there is a temperature gradient heat flows from high temperature to low…
Q: Two objects attract each other with a gravitational force of magnitude 9.40 10-9 N when separated by…
A:
Q: Consider a 3 kg object attached to a hanging spring with spring constant 75 N/m. Assume the system…
A: Given - Mass m=3kg Spring constant k=75NmExternal force Fe(t) = 10cos(5t) Initial displacement x(0)…
Q: An 1290 W toaster and an 803 W microwave oven are connected in parallel to the same 30.0 A, 120 V…
A:
Q: angular momentum Lis
A: There are several angular momentum operators, including:Total angular momentum: Usually denoted…
Q: A HeNe laser has a mode spacing of 500 MHz. Determine the cavity length.
A:
Q: A damaged 1310-kg car is being towed by a truck. Neglecting the friction, air drag, and rolling…
A: Mass Change in velocity Time Power
Q: Electricity and magnetism question: A point charge q is situated a large distance r from a neutral…
A: The final answer is attached below Explanation:
Q: A satellite is in a circular orbit with a radius of 6570 km and an inclination of 28°. It needs to…
A:
Q: A particular thermodynamic cycle acting on a monatomic ideal gas (y = 1.67) includes an isobaric…
A:
Needs Complete solution. Plz.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images
