Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 77QRT
Related questions
Question

Transcribed Image Text:Add Row 10 total rows
What is the reaction order?
0
1
02
Calculate the concentration of A after 163 s.
y = -0.03242 x + 3.257080
r² = 0.976
![For the reaction A → products, concentration and time data were collected.
Enter these data into the graphing tool to determine the reaction order.
t
Graphing Tool
0.0
25.0
50.0
73.0
[A]
3.40
2.28
1.53
1.02
In[A]
1.223775
0.824175
0.425268
0.019803
Clear All Data
1/[A]
0.294118
0.438596
0.653595
0.980392
[A]
3.4
2.822
2.244
1.666
1.088
0.51
[A] vs. t
to
0
14.6
t (s)
0.0
25.0
50.0
75.0
In[A] vs. t
29.2
t
43.8
1/[A] vs. t
58.4
[A] (M)
3.40
2.28
1.53
1.02
73](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc8271c0e-0172-414d-940a-e4ac3aaae156%2Fce6acb0f-389a-4291-af0e-4f9d76cf10d6%2Fadxqq8p_processed.png&w=3840&q=75)
Transcribed Image Text:For the reaction A → products, concentration and time data were collected.
Enter these data into the graphing tool to determine the reaction order.
t
Graphing Tool
0.0
25.0
50.0
73.0
[A]
3.40
2.28
1.53
1.02
In[A]
1.223775
0.824175
0.425268
0.019803
Clear All Data
1/[A]
0.294118
0.438596
0.653595
0.980392
[A]
3.4
2.822
2.244
1.666
1.088
0.51
[A] vs. t
to
0
14.6
t (s)
0.0
25.0
50.0
75.0
In[A] vs. t
29.2
t
43.8
1/[A] vs. t
58.4
[A] (M)
3.40
2.28
1.53
1.02
73
Expert Solution
Step 1
Well, we have to plot the data to confirm the order of the reaction-
the plot of graph for different order are as follow-
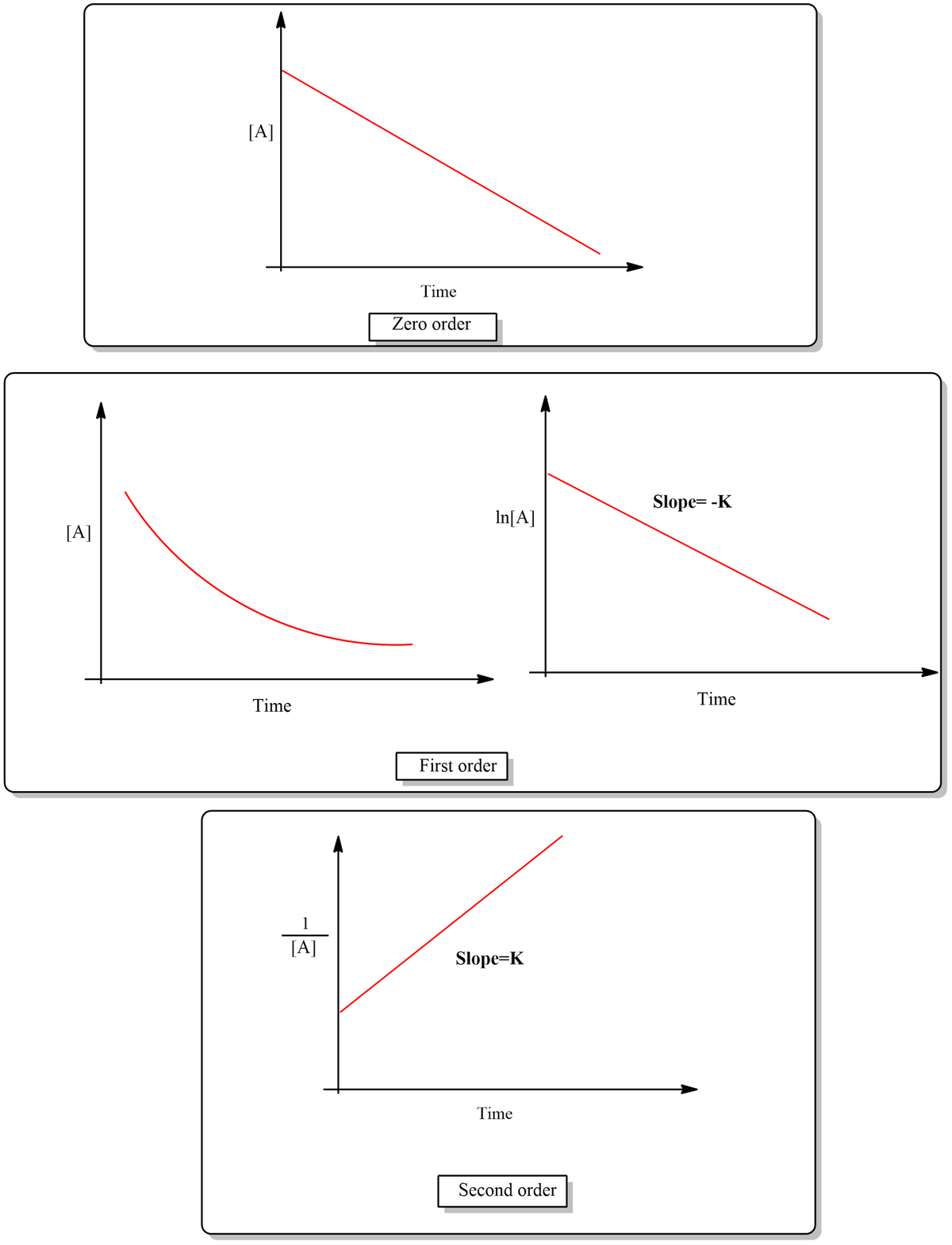
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning