
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.1, Problem 1P
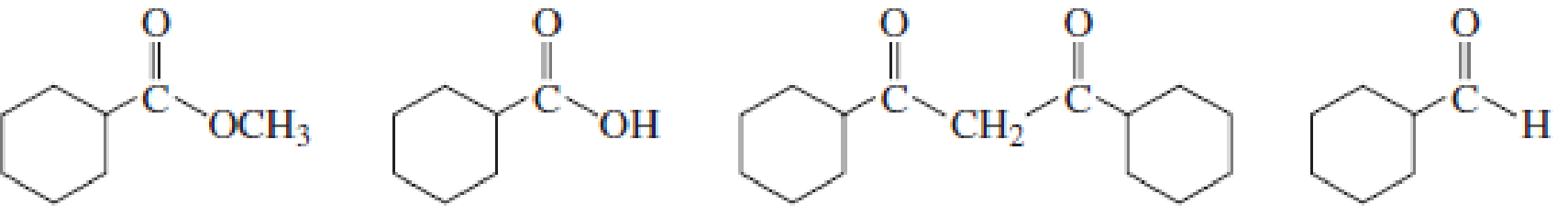
Identify the most acidic hydrogen in each compound.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
One of the three molecules is much more acidic than the other two. Identify the molecule
and explain why it is so much more acidic that the other two.
H
H
Rank the following compounds in terms of increasing acidity (least acidic first). Explain your ranking. Making sure to say which hydrogen in each molecule is the most acidic, and discuss the relative stability of the conjugate bases.
Select the most basic compound.
O H2S
OHCI
О H3P
Chapter 13 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 13.1 - Identify the most acidic hydrogen in each...Ch. 13.1 - Prob. 2PCh. 13.1 - Prob. 3PCh. 13.1 - Prob. 4PCh. 13.1 - Explain why HO cannot remove a proton from the...Ch. 13.2 - Prob. 6PCh. 13.2 - Prob. 7PCh. 13.3 - Prob. 8PCh. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.4 - Prob. 11PCh. 13.5 - Prob. 12PCh. 13.5 - Prob. 13PCh. 13.6 - Prob. 14PCh. 13.7 - Prob. 16PCh. 13.8 - Prob. 17PCh. 13.8 - Prob. 18PCh. 13.8 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.10 - Propose a mechanism for the formation of...Ch. 13.10 - Prob. 22PCh. 13.10 - a. If the biosynthesis of palmitic acid were...Ch. 13 - Draw the enol tautomers for each of the following...Ch. 13 - Number the following compounds in order from...Ch. 13 - Prob. 26PCh. 13 - Explain why the pKa of a hydrogen bonded to the...Ch. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Using cyclopentanone as the reactant, show the...Ch. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - Describe how the following compounds can be...Ch. 13 - Prob. 46PCh. 13 - Which would require a higher temperature:...Ch. 13 - Prob. 48PCh. 13 - Propose a mechanism for the following reaction:Ch. 13 - Show how the following compounds could be...Ch. 13 - Prob. 51PCh. 13 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Which is the most acidic hydrogen in the following compound? Provide a brief explanation. H H H. H, II IV +H `H.arrow_forwardWhich compound is the most acidic? Which compound is the least acidic? Rationalize acidity based on molecular structure.arrow_forwardAre acidic and basic rankings of the compounds correct?arrow_forward
- Explain why phenol is more acidic than alcohols by considering the resonance effect anddrawing the resonance structures of phenoxide ions.arrow_forwardFircle the compound with the most acidic hydrogen atom? SOH OH SMO CH || ||| 0716 NH₂ NH₂ IVarrow_forwardTenormin, a member of the group of drugs known as beta-blockers, is used to treat high blood pressure and improve survival after a heart attack. It works by slowing down the heart to reduce its workload. Which atom in Tenormin is the most basic?arrow_forward
- Complete each acid-base reaction and predict whether the position of equilibrium lies toward the left or toward the right. (a) CH3CCH+CH3CH2ONa+CH3CH3OH (b) CH3CCCH2CH2OH+Na+NH2NH3(l)arrow_forwardWhich of these four compounds is most acidic and whyarrow_forwardIdentify each reactant as an acid or a base, then draw the products that result from the provided arrows.arrow_forward
- Choose the most acidic and least acidic of the compounds given below. A OH B The most acidic compound is: [Select] The least acidic compound is: [Select] OH C OH CI D ū O OHarrow_forwardProvide the pH values for the following 4 compounds.arrow_forwardplease draw resonance structures for both and explain which one is the strongest acid based on the structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY