
Concept explainers
Figure 2.3 How many neutrons do carbon-12 and carbon-13 have, respectively?
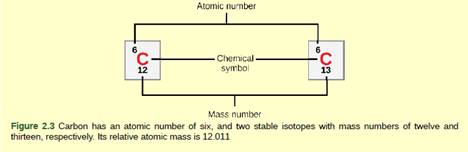
To write:
Number of neutrons in carbon-12 and carbon-13 respectively.
Introduction:
An atom consists of subatomic particles which are electrons, protons and neutrons. Every element in the periodic table has a different mass number and atomic number and based on that they have different number of subatomic particles. An atom consists of a nucleus which is composed of protons and neutrons. Neutrons as the name define; they are neutral in nature which means they don’t possess any electrical charge.
Explanation of Solution
The number of protons and neutrons in an atom of an element is called as its mass number and number of protons in an element is called as its atomic number. To calculate the number of neutrons, we need to subtract no of protons from the mass number.
Want to see more full solutions like this?
Chapter 2 Solutions
Biology 2e
Additional Science Textbook Solutions
College Physics
Microbiology with Diseases by Body System (5th Edition)
Biology: Life on Earth (11th Edition)
Human Biology: Concepts and Current Issues
Campbell Biology (11th Edition)
Microbiology: An Introduction
- Figure 2.3 How many neutrons do (K) potassium-39 and potassium-40 have, respectively?arrow_forwardPolonium is a rare element with 33 radioisotopes. The most common one, 210Po, has 82 protons and 128 neutrons. When 210Po decays, it emits an alpha particle, which is a helium nucleus (2 protons and 2 neutrons). 210Po decay is tricky to detect because alpha particles do not carry very much energy compared to other forms of radiation. For example, they can be stopped by a single sheet of paper or a few inches of air. That is one reason that authorities failed to discover toxic amounts of 210Po in the body of former KGB agent Alexander Litvinenko until after he died suddenly and mysteriously in 2006. What element does an atom of 210Po become after it emits an alpha particle?arrow_forwardWrite the nuclear equation for the positron decay of C-11.arrow_forward
- 238. 234. 14) Uranium-238 ( 92") decays to form thorium-234 ( 90 h) with a half-life of 4.5 x 10° years. How many years will it take for 75% of the uranium-238 to decay? а) 9.0х 10 years с) 9.0х10° years b) 4.5 x 10° years d). 3.8 х 10° усаrsarrow_forwardWhat subatomic particle do all Carbon atoms, isotopes and ions have in common?arrow_forwardAssume that you list the following types of electromagnetic radiation in order of increasing wavelength: () the gamma rays produced by a radioactive nuclide used in medical imaging: (ii) radiation from an FM radio station at 93.1 MHz on the dial; (iii) a radio signal from an AM radio station at 680 kHz on the dial; (iv) the yellow light from sodium vapor streetlights; (v) the red light of a light-emitting diode. Which one would be the second? Lütfen birini seçin: O a 680 kHz AM radio waves O b. the red light O c the yellow light O d. 93.1 MHz FM radio waves O e the gamma raysarrow_forward
- How can a nucleus emit an electron during B decay when there are no electrons present in the nucleus to begin with?arrow_forwardIodine has 37 known isotopes. Therefore, the atomic mass has a range of 108-144 amu. Which of the following statements concerning iodine is correct? A) The isotopes of iodine have between 55 and 91 protons. B) An atom of iodine can have between 55 and 91 neutrons. C) The isotopes of iodine will always have the same number of neutrons, but the protons can vary. D) The isotopes of iodine have between 108 and 144 neutrons, but the number of protons will not vary.arrow_forwardThe half-life of 1311 is 8.04 days. (a) Convert the half-life to seconds. (b) Calculate the decay constant for this isotope. s-1 (c) Convert 0.550 µCi to the SI unit the becquerel. |Bq (d) Find the number of 1311 nuclei necessary to produce a sample with an activity of 0.550 μCi. | 1311 nuclei (e) Suppose the activity of a certain 131I sample is 7.10 mCi at a given time. Find the number of half-lives the sample goes through in 40.2 d and the activity at the end of that period. (Enter your answer for the number of half-lives to at least one decimal place.) half-lives mCiarrow_forward
- Carbon 14 is an isotope of Carbon-12. What makes Carbon 14 an isotope?arrow_forwardIf there is 10 μmol of the radioactive isotope 32P (half-life 14 days) at t = 0, how much 32P will remain at (a) 7 days, (b) 14 days, (c) 21 days, and (d) 70 days?arrow_forwardFor Be-10, find the: a.) mase defect (in grams) b.) binding energy in kilojoules per mole. mass proton= 1.00728 amu; mass neutron= 1.00867 amu; mass Be-10 = 10.013534679 amuarrow_forward
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning





