An exothermic reaction with a 1-to-1 stoichiometric ratio and known enthalpy, DHrxn = -3.45 kJ/mol, is performed in a constant pressure calorimeter. When 0.592 mol of the limiting reagent reacted, the temperature of the calorimeter increased from 293.15 K to 296.89 K.
An exothermic reaction with a 1-to-1 stoichiometric ratio and known enthalpy, DHrxn = -3.45 kJ/mol, is performed in a constant pressure calorimeter. When 0.592 mol of the limiting reagent reacted, the temperature of the calorimeter increased from 293.15 K to 296.89 K.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.63PAE: 9.63 Reactions of hydrocarhons are often studied in the petroleum industry. One example is...
Related questions
Question
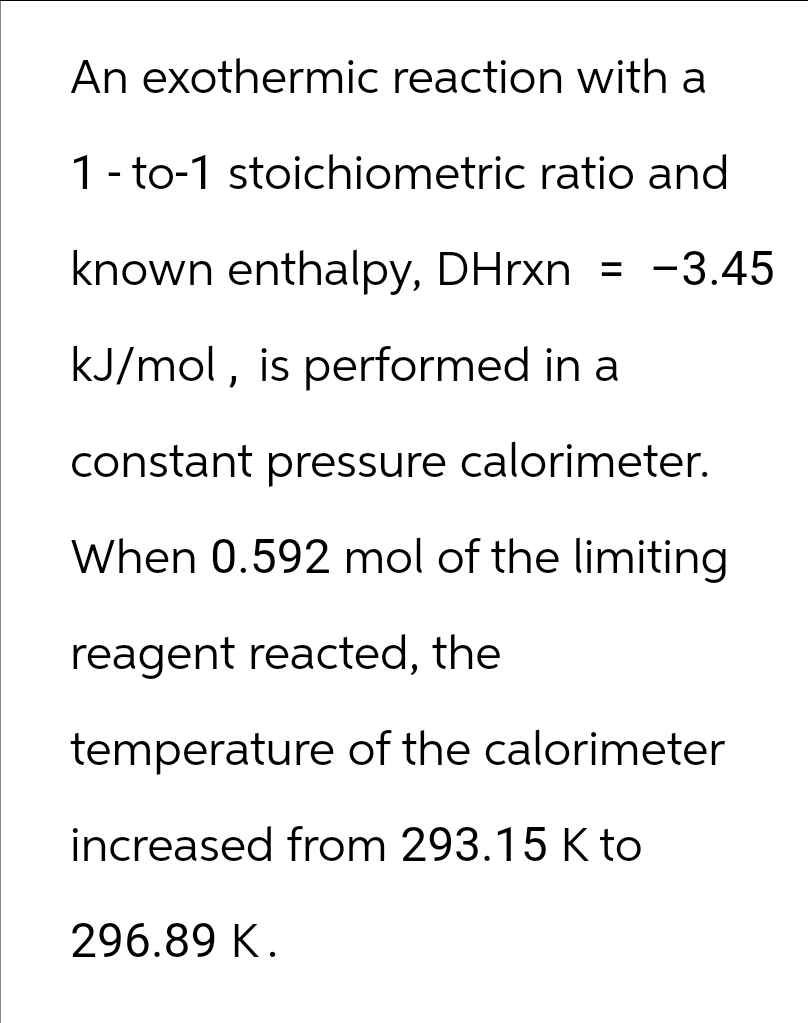
Transcribed Image Text:An exothermic reaction with a
1-to-1 stoichiometric ratio and
known enthalpy, DHrxn = -3.45
kJ/mol, is performed in a
constant pressure calorimeter.
When 0.592 mol of the limiting
reagent reacted, the
temperature of the calorimeter
increased from 293.15 K to
296.89 K.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning