
Concept explainers
(a)
Interpretation:
Lewis structure for the
Concept introduction:
Lewis structure is a convenient way to convey information such as which atoms are bonded to each other by which type of bond. Only valence electrons take part to draw Lewis structure.
Valence electrons that participate in bond formation are called bonding electron pairs whereas electrons that do not take part in bonding and that remain as non-bonding electrons are termed as lone pair of electrons.
Lewis structure is drawn from the total valence electron count of each atom in the molecule. Skeleton structure for the given molecule is drawn with atoms bonded with a single bond, the central atom is always less electronegative atom. Electrons that participate in forming the bond are bonding electrons. Remaining electrons are distributed first to outer atoms such that each atom completes its octet other than hydrogen.
Answer to Problem 1.44P
Lewis structure for
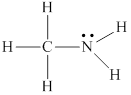
Explanation of Solution
The given molecule
Thus the skeleton structure for
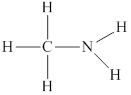
Remaining two valence electrons are contributed as lone pair of electrons to the outer atom which is nitrogen.
Hence the complete Lewis structure for

Lewis structure for
(b)
Interpretation:
Lewis structure for
Concept introduction:
Lewis structure is drawn from the total valence electron count of each atom in the molecule. Skeleton structure for the given molecule is drawn with atoms bonded with a single bond, the central atom is always less electronegative atom. Electrons that participate in forming the bond are bonding electrons. Remaining electrons are distributed first to outer atoms such that each atom complete its octet other than hydrogen.
Answer to Problem 1.44P
Lewis structure for
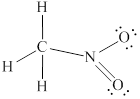
Explanation of Solution
In
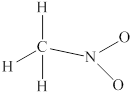
Remaining
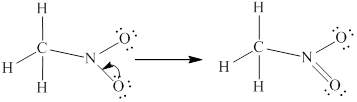
Lewis structure for
(c)
Interpretation:
Lewis structure for
Concept introduction:
Lewis structure is drawn from the total valence electron count of each atom in the molecule. Valence electrons participate in bond formation are called bonding electron pairs whereas electrons that do not take part in bonding and that remain as non-bonding electrons are termed as lone pair of electrons. Skeleton structure for the given molecule is drawn with atoms bonded with a single bond, the central atom is always less electronegative atom.
Lone pair of electrons contributed to outer atoms to complete octet except hydrogen.
Answer to Problem 1.44P
The Lewis structure for
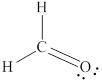
Explanation of Solution
Carbon is the central atom in
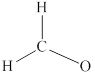
Remaining six electrons are contributed as lone pairs to the outer atom oxygen but one electron is involved in bonding with complete octet of oxygen atom.
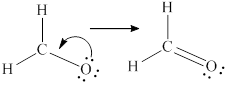
Lewis structure is drawn from the total valence electron count.
(d)
Interpretation:
Lewis structure for
Concept introduction:
Lewis structure is drawn from the total valence electron count of each atom in the molecule. Skeleton structure for the given molecule is drawn with atoms bonded with a single bond, the central atom is always less electronegative atom. Electrons that participate in forming the bond are bonding electrons. Remaining electrons are distributed first to outer atoms such that each atom completes its octet other than hydrogen.
Answer to Problem 1.44P
Lewis structure for
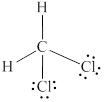
Explanation of Solution
In
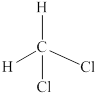
Eight electrons from total
Therefore the Lewis structure for

Lewis structure is drawn from total valence electron count.
(e)
Interpretation:
Lewis structure for
Concept introduction:
Lewis structure is drawn from the total valence electron count of each atom in the molecule. Valence electrons that participate in bond formation are called bonding electron pairs whereas electrons that do not take part in bonding and that remain as non-bonding electrons are termed as lone pair of electrons. Skeleton structure for the given molecule is drawn with atoms bonded with a single bond, the central atom is always less electronegative atom.
Lone pair of electrons contributed to outer atoms to complete octet except hydrogen.
Answer to Problem 1.44P
Lewis structure for
![]()
Explanation of Solution
The given molecule
![]()
Remaining
Hence the Lewis structure for
![]()
Lewis structure is drawn from total valence electron count.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- (a) Draw Lewis structure of the following: (i) H3PO4 (ii) N3 - (b) How many hydrogen atoms are present in 10 g of sugar? (c) Calculate formal charge of each atom of CH3O-arrow_forwardDraw a Lewis Structure for each of the following species and assign formal charge where appropriate. Using electronegative values from the period table that was provided identify polar covalent bonds and label the atoms δ+ and δ−. For each of the molecules indicate whether or not it has a dipole moment. (a)CH5N (b) HCN (c) H2CO (d) CH3NC(e) CH3SOCH3 (f) H6BNarrow_forwardWrite Lewis formulas, including unshared pairs, for each of the following. Carbon has four bonds in each compound. (a) Propane (C3H8) (c) Methyl fluoride (CH3F) (b) Methanol (CH4O) (d) Ethyl fluoride (C2H5F)arrow_forward
- Arrange each set of bonds in order of increasing polarity, and indicate bond polarity with δ+and δ- symbols: (a) Cl-F, Br-Cl, Cl-Cl (b) P-F , Si-F, S-F and Arrange each set of bonds in order of decreasing polarity, and indicate bond polarity with a polar arrow: (a) Se-Cl, Se-F, Se-Br (b) S-B, F-B, Cl-Barrow_forwardJudging from their relative positions in the Periodic Table, which atom in each set is more electronegative? (a) Carbon or nitrogen (b) Chlorine or bromine (c) Oxygen or sulfurarrow_forwardUsing the bond energies as shown, determine the approximate enthalpy change for each of the following reactions:(a) Cl2(g) + 3F2(g) ⟶ 2ClF3(g)(b) H2 C = CH2(g) + H2(g) ⟶ H3 CCH3(g)(c) 2C2 H6(g) + 7O2(g) ⟶ 4CO2(g) + 6H2 O(g)arrow_forward
- Draw Lewis structures for the following compounds and ions, showing appropriateformal charges.(a) [CH3OH2 ]+ (b) NH4Cl (c) (CH3)4NCl(d) NaOCH3 (e) +CH3 (f) -CH3(g) NaBH4 (h) NaBH3CN (i) (CH3)2O¬BF3(j) [HONH3]+ (k) KOC(CH3)3 (l) [H2C“OH]arrow_forwardPick the correct and preferable Lewis structure of H3NO H-Ñ-H :Ö-H :Ñ-H :ö: (a) 0: (b) H-Ñ: (c) H-Ó: (d) H-N-H Structure (a) Structure (b) Structure (c) Structure (d)arrow_forwardWhich bond in each of the following pairs of bonds is the strongest?(a) C–C or C = C(b) C–N or C ≡ N(c) C ≡ O or C = O(d) H–F or H–Cl(e) C–H or O–H(f) C–N or C–Oarrow_forward
- For each of the following covalent bonds: (a) use the symbols δ+ and δ- to indicate the direction of polarity (if any).(a) C-F; (b) N-Br; (c) B-C; (d) Si-H(b) Rank the following covalent bonds in order of increasing polarity. (i) C-H, O-H, N-H; (ii) C-N, C-O, B-O; (iii) C-P, C-S, C-Narrow_forwardWhich of the following bonds is the shortest in length? (a) C-O (b) C-Si (c) 0=0 (d) C-Brarrow_forward(a) Complete the Lewis structure for vinyl chloride by showing all unshared pairs of electrons. (b) Predict the H-C-H, H-C-C, and Cl-C-H bond angles in this molecule. (c) Does vinyl chloride have polar bonds? Is it a polar molecule? Does it have a dipole?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





