
Concept explainers
For a particle in a state having the wavefunction
(a)
(c)
(e)
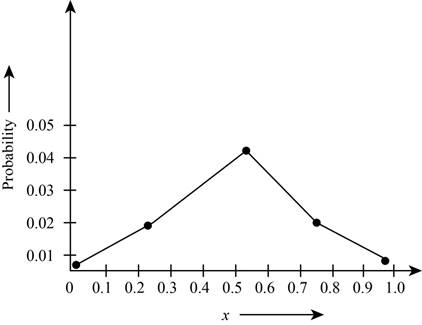
Plot the probabilities versus
(a)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.26E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above expression is simplified as follows.
The probability for the particle having wavefunction
(b)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.26E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above expression is simplified as follows.
The probability for the particle having wavefunction
(c)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.26E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above expression is simplified as follows.
The probability for the particle having wavefunction
(d)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.26E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above expression is simplified as follows.
The probability for the particle having wavefunction
(e)
Interpretation:
The probability for the particle having wavefunction
Concept introduction:
For the normalization of the wavefunction, the wavefunction is integrated as a product of its conjugate over the entire limits. It is expressed by the equation as given below.
Where,
•
•
•
Answer to Problem 10.26E
The probability for the particle having wavefunction
Explanation of Solution
For the probability of the wavefunction the expression is as follows.
Where,
•
•
•
•
Substitute the values in the above equation as follows.
The above expression is simplified as follows.
Theplot the probabilities versus

Figure 1
The plot shows the probability for the given wave function. According to this plot, the probability of finding the particle is maximum in the range of
The probability for the particle having wavefunction
Want to see more full solutions like this?
Chapter 10 Solutions
Physical Chemistry
- Imagine a particle free to move in the x direction. Which of the following wavefunctions would be acceptable for such a particle? In each case, give your reasons for accepting or rejecting each function. (1) Þ(x) = x²; (iv) y(x) = x 5. (ii) ¥(x) = ; (v) (x) = e-* ; (iii) µ(x) = e-x²; (vi) p(x) = sinxarrow_forwardImagine a particle free to move in the x direction. Which of the following wavefunctions would be acceptable for such a particle? In eachcase, give your reasons for accepting or rejecting each function. (i) Ψ(x)=x2; (ii) Ψ(x)=1/x; (iii) Ψ(x)=e-x^2.arrow_forwardThe ground-state wavefunction for a particle confined to a one dimensional box of length L is Ψ =(2/L)½ sin (πx/L) Suppose the box 10.0 nm long. Calculate the probability that the particle is: (a) between x = 4.95 nm and 5.05 nm (b) between 1.95 nm and 2.05 nm, (c) between x = 9.90 and 10.00 nm, (d) in the right half of the box and (e) in the central third of the box.arrow_forward
- For a particle in the stationary state n of a one dimensional box of length a, find the probability that the particle is in the region 0 xa/4. (b) Calculate this probability for n = 1, 2, and 3arrow_forwardA normalized wavefunction for a particle confined between 0 and L in the x direction is ψ = (2/L)1/2 sin(πx/L). Suppose that L = 10.0 nm. Calculate the probability that the particle is (a) between x = 4.95 nm and 5.05 nm, (b) between x = 1.95 nm and 2.05 nm, (c) between x = 9.90 nm and 10.00 nm, (d) between x = 5.00 nm and 10.00 nm.arrow_forward(a) For a particle in the stationary state n of a one dimensional box of length a, find the probability that the particle is in the region 0 x a/4. (b) Calculate this probability for n = 1, 2, and 3 Sketch and | |2 for the n = 4 and n = 5 states of a particle in a one-dimensional box.arrow_forward
- 106. Combining two real wave functions ₁ and 2, the following functions are constructed: A = ₁ + $₂₂ B = = ₁ +i0₂, C = ₁ −i0₂, D=i(0₁ +0₂). The correct statement will then be (a) A and B represent the same state (c) A and D represents the same state (b) A and C represent the same state. (d) B and D represent the same state.arrow_forwardThe given wave function for the hydrogen atom is y =w,00 +210 + 3y2 · Here, ypim has n, 1, and m as principal, orbital, and magnetic quantum numbers respectively. Also, yim an eigen function which is normalized. The expectation value of L in the state wis, is 9h? (a) 11 (b) 11h? 20 (c) 11 (d) 21ħ?arrow_forwardIf two wavefunctions, Wa and Wb, are orthonormal and degenerate, then what is true about the linear combinations 1 1 w. +v.) a a and (a) y+ and y- are orthonormal. (b) y+ and y- are no longer eigenfunctions of the Schrödinger equation. (c) V+ and y- have the same energy. (d) V+ and Y- have the same probability density distribution.arrow_forward
- The ground state wave function for a particle in a one-dimensional box is of length L is y = (2/L)¹² sin(7x/L). Calculate the probability of the particle between x=4.00 nm to x = 4.80 nm. Assume the length of the box is 8.5 nm. Answer Choices: (A) 0.840 (B) 0.143 (C) 0.186 (D) 0.256arrow_forward(a) If  = 3x? and B = , then show that  and ß donot commute with respect to the function f(x) = sin x. Show, if the wave function, w) = A cos(kx) + iA sin(kx) is an Eigen-function of the linear momentum operator, P and if so, what is the Eigen value. (Note: A and k are constants). (b)arrow_forward8C.4 (a) the moment of inertia of a CH4 molecule is 5.27 x 10^-47 kg m^2. What is the minimum energy needed to start it rotating? 8C.5 (a) use the data in 8C.4 (a) to calculate the energy needed excite a CH4 molecule from a state with l=1 to a state with l=2arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
