
a)
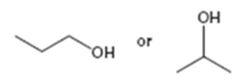
Interpretation:
Among the two alcohols, 1-propanol and 2-propanol, which will react faster with HX to form the corresponding
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 1-propanol and 2-propanol, which will react faster with HX to form the corresponding alkyl halide.
b)
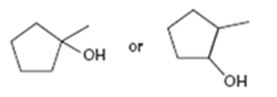
Interpretation:
Among the two alcohols, 1-methylcyclopentanol and 2-methylcyclopentanol, which will react faster with HX to form the corresponding alkyl halide is to be stated.
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 1-methylcyclopentanol and 2-methylcyclopentanol, which will react faster with HX to form the corresponding alkyl halide.
c)
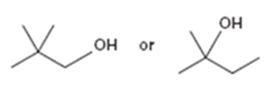
Interpretation:
Among the two alcohols, 2,2-dimethyl-1-propanol and 2-methyl-2-butanol, which will react faster with HX to form the corresponding alkyl halide is to be stated.
Concept introduction:
Alcohols react with HX to yield the corresponding alkyl halide. The order of reactivity is methyl < primary < secondary < tertiary.
To state:
Among the two alcohols, 2,2-dimethyl-1-propanol and 2-methyl-2-butanol, which will react faster with HX to form the corresponding alkyl halide.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry
- Draw the products of each reaction. Assume excess halogen is present.arrow_forward2. The following carbocation is generated as an intermediate in the addition of H-Br to an alkene. Draw the structure of all possible alkenes that could have formed this intermediate.arrow_forwardComplete the following reactions in sequential order. Show the product after each step and put a box around the final product. (Show intermediate structure after each reagent)arrow_forward
- Identify the nucleophile and leaving group and draw the products of each substitution reaction.arrow_forwardAddition of water to an alkyne gives a keto‑enol tautomer product. Draw the ketone that is in equilibrium with the given enol.arrow_forwardDraw the major 1,2- and 1,4-addition products obtained in the reaction shown. Assume that both are derived from the most stable carbocation intermediate. HBrarrow_forward
- . Which of the following can be both formed from bromoethane and converted directly into ethanal? CH3CH2Br → X → CH3CHO a. CH3CH2OH b. CH3OCH3 c. CH3COOH d. H2C=CHBrarrow_forwardDraw the resulting product/s of the given reactions.arrow_forwardDraw the major organic product formed by reaction of 2-hexyne with the following reagent: H₂O in H₂SO4 / HgSO4. • Consider E/Z stereochemistry of alkenes. • In cases where there is more than one answer, just draw one. • If no reaction occurs, draw the organic starting material.arrow_forward
- Draw a major resonance contributor of this enolate anion. Include all lone pairs in your structure. Θ 0:☀ :0:arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. LOCH3 LOCH3 HNO3 / CH3CO₂H O₂N • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. ● In cases where there is more than one answer, just draw one.arrow_forwardFunctional groups such as alkynes react the same in complex molecules as they do in simpler structures. The following example of alkyne reaction were taken from syntheses carried out in the research group of E. J. Corey at Harvard University. You can assume that the reactions listed involve only the alkyne, not any of the functional groups present in the molecules. Draw the expected products for the following reaction .arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

