
Concept explainers
(a) The structure of
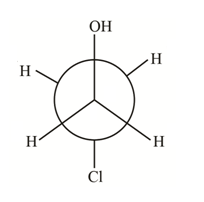
How many distinct
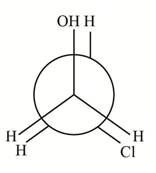
How many distinct
Trending nowThis is a popular solution!

Chapter 16 Solutions
Physical Chemistry
- The following 1H NMR peaks were recorded on a spectrometer operating at 200 MHz. Convert each into δ units. (a) CHCl3; 1454 Hz (b) CH3Cl; 610 Hz (c) CH3OH; 693 Hz (d) CH2Cl2; 1060 Hzarrow_forward13C(1h) what is bracket mean in this case? is that mean carbon nmr proton nmr in same time?arrow_forwardThe following molecule will give two signals in the proton (¹H) NMR. H₂C What is the ratio of the signal intensities for the two peaks? 2:2 O 3:1 3:2 CH₂ O 2:1arrow_forward
- For the following compound how many different signals would you see in the carbon NMR? (Assume that you can see them all.) 4 O 5 O 3 09 CO |arrow_forwardOrganic Chemistry - How many signals would you expect in the 1H NMR spectrum of HOCH2CH2CH2CH2OH?arrow_forward(a) What would be the chemical shift of a peak that is observed at 655.2 Hz from the reference tetramethylsilane (TMS) recorded using a 90 MHz spectrometer ? (b) At what frequency would the chemical shift of chloroform (CHCl3, δ = 7.28 ppm) occur relative to TMS on a spectrum recorded on a 300 MHz spectrometer? (c) At what frequency and chemical shift would the signal for chloroform occur when using a 1 GHz NMR spectrometer?arrow_forward
- Fill in the Blanks Type your answers in all of the blanks and submit X₂ X² Ω· Determine the number of signals in the ¹H-NMR spectrum of the given compound as well as the ratio of its integrated signals. Number of signals: 7 Integration: 1 You are incorrect You are incorrect X X (express as the lowest whole number ratio from smallest to largest, e.g.,arrow_forwardA student needs to prepare a ¹H NMR sample for an organic compound soluble in chloroform (CHCI3). The student cannot use CHCI 3 as the solvent for the sample, but rather uses deuterated chloroform (CDC13) because: O CDC13 is more polar. O CDC13 will not produce a signal in the ¹H NMR spectrum, and therefore will not mask the signals of the compound. O The compound will be more soluble in CDCI 3. O CDC13 is cheaper than CHCI 3.arrow_forwardSketch the form of an A3M2X4 spectrum, where A, M, and X are protons with distinctly different chemical shifts and JAM > JAX > JMX ·arrow_forward
- Propanone (acetone, (CH3)2CO) has a strong absorption at 189 nm and a weaker absorption at 280 nm. Identify the chromophore and assign the absorptions to π* ← n or π* ← π transitions.arrow_forward(Figure 1) Figure (a) (c) (CH3)3C H (b) (d) 1 of 1 Part A Predict the number of 13C NMR signals in the proton-decoupled spectrum of (a). Express your answer numerically using one significant figure. 15| ΑΣΦ Part B ? Predict the number of 13C NMR signals in the proton-decoupled spectrum of (b). Express your answer numerically using one significant figure. ΠΫΠΙ ΑΣΦ ?arrow_forwardhow many peaks wouldyou expect to see in the 13CNMR spectrum of this substance ?arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

