
(a)
Interpretation:
The name of the organic compound formed by elimination from an alcohol needs to be determined.
Concept introduction:
In a substitution reaction, one
Explanation of Solution
Alcohol undergoes elimination reaction to form
For example:
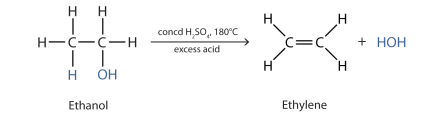
In the above reaction, ethanol reacts with concentrated sulfuric acid to form alkene and water.
(b)
Interpretation:
The name of the organic compound formed by addition of hydrogen chloride to an alkene needs to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
The addition reaction of hydrogen chloride to alkene results in the formation of
For example:
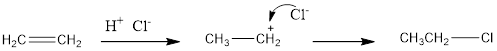
(c)
Interpretation:
The name of the organic compound formed by addition of water to an alkene needs to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
The addition of water to alkene in the presence of an acid results in the formation of an alcohol. This is known as hydration of an alkene.
For example:
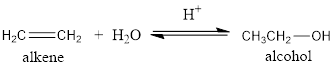
(d)
Interpretation:
The name of the organic compound formed by substitution of a hydroxyl group for a halogen atom needs to be determined.
Concept introduction:
In a substitution reaction, one functional group is replaced by other functional group. In addition reaction, two reactants simply add to form a single product. In condensation reaction, addition of two reactants takes place after removal of water molecule. The elimination reaction results when removal of two substituent groups takes place from a molecule.
Explanation of Solution
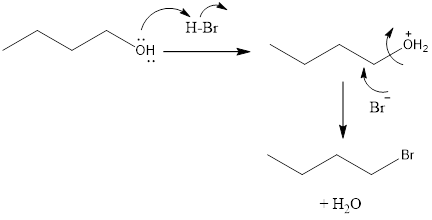
The substitution of hydroxyl group for a halogen atom results in the formation of an alkyl halide from an alcohol. This results in the formation of water molecule as a byproduct.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Chemistry: The Central Science (13th Edition)
Essential Organic Chemistry (3rd Edition)
Inorganic Chemistry
Chemistry: A Molecular Approach
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





