
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 63P
Interpretation Introduction
Interpretation:Out of the three chlorinated compounds, one that does not give the indicated
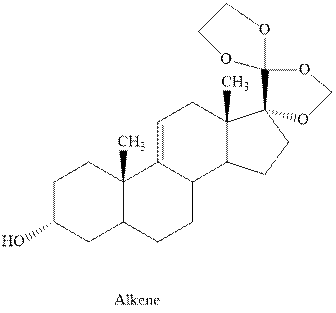
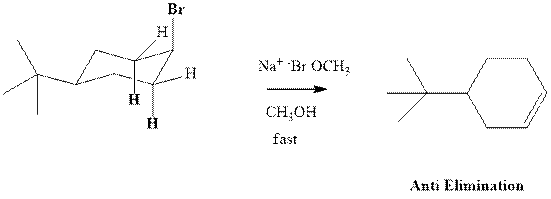
Concept introduction: Experimental evidence for
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Trimethyl Phosphite reacts with bromomethane to afford the product shown below via a two-step mechanism. Draw a detailed mechanism for this reaction using arrows to show electron flow (both mechanistic steps are SN2 reactions).
Although there are nine stereoisomers of 1,2,3,4,5,6-hexachlorocyclohexane, one stereoisomer reacts 7000 times more slowly than any of the others in an E2 elimination. Draw the structure of this isomer and explain why this is so.
Compounds X and Y are both C7H15Cl products formed in the radical chlorination of 2,4-dimethylpentane.
Base-promoted E2 elimination of X and Y gives, in each case, a single C7H₁4 alkene.
Both X and Y undergo an SN2 reaction with sodium iodide in acetone solution to give C7H15l products; in this reaction Y reacts faster than X.
What is the structure of X?
• Do not use stereobonds in your answer.
• In cases where there is more than one possible structure for each molecule, just give one for each.
.
Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Separate structures with + signs from the drop-down menu.
на
Chapter 7 Solutions
Organic Chemistry: Structure and Function
Ch. 7.1 - Prob. 7.1ECh. 7.2 - Prob. 7.2ECh. 7.3 - Prob. 7.3ECh. 7.3 - Prob. 7.5TIYCh. 7.4 - Prob. 7.7TIYCh. 7.5 - Prob. 7.9TIYCh. 7.6 - Prob. 7.10ECh. 7.7 - Prob. 7.11ECh. 7.7 - Prob. 7.12ECh. 7.7 - Prob. 7.13E
Ch. 7.7 - Prob. 7.15TIYCh. 7.8 - Prob. 7.16ECh. 7.8 - Prob. 7.17ECh. 7.9 - Prob. 7.18ECh. 7.9 - Prob. 7.19ECh. 7.9 - Prob. 7.20ECh. 7.9 - Prob. 7.21ECh. 7.9 - Prob. 7.22ECh. 7 - Prob. 25PCh. 7 - Prob. 26PCh. 7 - Prob. 27PCh. 7 - Prob. 28PCh. 7 - Prob. 29PCh. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Prob. 32PCh. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Prob. 39PCh. 7 - Prob. 40PCh. 7 - Prob. 41PCh. 7 - Prob. 42PCh. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Prob. 45PCh. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - Prob. 48PCh. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Prob. 55PCh. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Prob. 59PCh. 7 - Prob. 60PCh. 7 - Prob. 61PCh. 7 - Prob. 62PCh. 7 - Prob. 63PCh. 7 - Prob. 64PCh. 7 - Prob. 65PCh. 7 - Prob. 66PCh. 7 - Prob. 67PCh. 7 - Prob. 68PCh. 7 - Prob. 69PCh. 7 - Prob. 70P
Knowledge Booster
Similar questions
- How will you synthesize cyclohexanecarboxaldehyde (cyclohexylmethanal) from the following reagents? (There are no restrictions on the reagents or the number of steps). (a) Cyclohexanone (b) Ethynylcyclohexane (c) Methyl cyclohexylformate (Remember: Formic acid is the IUPAC recognized name for Methanoic acid) (d) Cyclohexanecarboxylic acid (Cyclohexylmethanoic acid) (e) Vinylcyclohexanearrow_forwardIdentify products A and B from the given 1H NMR data. Treatment of acetone [(CH3)2C=O] with dilute aqueous base forms B. Compound B exhibits four singlets in its 1H NMR spectrum at 1.3 (6 H), 2.2 (3 H), 2.5 (2 H), and 3.8 (1H) ppm. What is the structure of B?arrow_forwardWhat product or products are produced in each of the following reactions? By what mechanism ( SN1, SN2,E1 or E2 ) does each react? Show the resulting products as single product, main product and by-product.arrow_forward
- A chemist needs an ether to use as a solvent for a reaction and wants to synthesize it in one step from two of the following available reagents: sodium ethoxide, bromomethane, potassium tert-butoxide, and 2-bromo-2-methylpropane. i) Which combination(s) will give a good yield of an ether? Illustrate, showing the mechanism of the reaction. ii) Illustrate with a mechanism the reaction of one of the combinations that will not yield an ether?arrow_forwardIllustrate the resonance effect of the methoxy group -OCH3, on the structure of the benzene ring. Draw all the oissuvke resonance forms of methoxybenzene, including the hybrid Based on the structures, explain how the presence of the -OCH3 group affects: (i) the reactivity of the benzene ring towards electrophilic attack (ii) the orientation or point of attack of an incoming electrophilic reagent on the benzene ring.arrow_forwardWhen 2-bromo-3-phenylbutane is treated with sodium methoxide, two alkenes result (by E2 elimination). The Zaitsevproduct predominates.(a) Draw the reaction, showing the major and minor products.(b) When one pure stereoisomer of 2-bromo-3-phenylbutane reacts, one pure stereoisomer of the major product results.For example, when (2R,3R)-2-bromo-3-phenylbutane reacts, the product is the stereoisomer with the methyl groups cis.Use your models to draw a Newman projection of the transition state to show why this stereospecificity is observed.(c) Use a Newman projection of the transition state to predict the major product of elimination of (2S,3R)-2-bromo-3-phenylbutanearrow_forward
- Which of these structures fit the following descriptions? Br An alkyl halide that gives a mixture of three alkenes on E2 reaction H An organohalide that will not undergo nucleophilic substitution An alkyl halide that gives the non-Zaitsev product on E1 reaction CH2CH2 An alcohol that reacts rapidly with HCI at 0° (CH3};COHarrow_forwardWhen 2-bromo-3-phenylbutane is treated with sodium methoxide, two alkenes result (by E2 elimination). The Zaitsevproduct predominates.(a) Draw the reaction, showing the major and minor products.(b) When one pure stereoisomer of 2-bromo-3-phenylbutane reacts, one pure stereoisomer of the major product results.For example, when (2R,3R)-2-bromo-3-phenylbutane reacts, the product is the stereoisomer with the methyl groups cis.Use your models to draw a Newman projection of the transition state to show why this stereospecificity is observedarrow_forwardAlthough diazomethane (CH2N2) is often not a useful reagent for preparing cyclopropanes, other diazo compounds give good yields of more complex cyclopropanes. Draw a stepwise mechanism for the conversion of diazo compound A to B, an intermediate in the synthesis of sirenin, the sperm attractant produced by the female gametes of the water mold Allomyces.arrow_forward
- Which sequence of reactions will convert benzene to 3-chloroaniline in a reasonable yield? (Hint: a nitro group can be reduced to an amine group)arrow_forwardCompound A on ozonolysis yields the two products shown. What is the structure of compound A? Compound A 1.03 2. (CH3)2S ||| ol H H || IV H Harrow_forwardBoth p-chloronitrobenzene (1) and p-chloroanisole (2) react with sodium ethoxide in ethanol, however one substrate requires much stronger heating than the other. Also, two substitution products are obtained from one of the substrates whereas only one substitution product is obtained from the other. Explain these facts mechanistically. O₂N 1 > CI CH3O 2 > CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning