
Concept explainers
Ethanol,
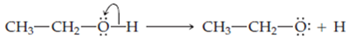
In this structure the oxygen owns one electron from
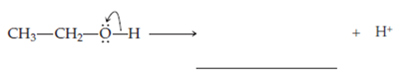
Note that the other fragment, the proton, leaves with a formal charge of +1.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Pushing Electrons
- The n-propyl cation can be formed from a molecule such as When the C–Cl bond is broken so that both electrons leave with Cl, the fragments formed are The carbon atom that had been attached to Cl is now sharing ____ electron pairs. In each shared pair the carbon atom owns ____ electron. The number of electrons that belong to carbon is ____. The formal charge on the carbon atom is ____. The correct Lewis structure for the n-propyl cation isarrow_forwardWrite all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardThe chemistry of the nitrite ion and HNO2: (a) Two resonance structures are possible for NO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. (b) In forming the acid HNO2 an H+ ion attaches to the O atom and not the N atom of NO2. Explain why you would predict this result. (c) Two resonance structures are possible for HNO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. Is either of these structures strongly preferred over the other?arrow_forward
- The electrostatic potential surface for SOCl2 is pictured here. (a) Draw a Lewis electron dot picture for the molecule, and give the formal charge of each atom. (b) What is the molecular geometry of SOCl2? Is it polar?arrow_forwardChloromethane has the Lewis structure _______________________________ The carbon atom is sharing 4 electron pairs. In each shared pair the carbon atom “owns” 1 electron. The number of electrons that “belong” to carbon is ___. Carbon, being a Group ___ element would have 4 , outer shell electrons in the unbonded, neutral state. Therefore, the carbon atom in chloromethane has a formal charge of zero.arrow_forwardA common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete Lewis structure for an organic compound called methyl cyanoacrylate, the main ingredient in Super Glue. Draw a complete Lewis structure for methyl cyanoacrylate in which all atoms have a formal charge of zero.arrow_forward
- Write the correct Lewis structure and assign a formal charge to each atom in fulminate ion, CNO.arrow_forwardFormamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardWrite Lewis structures for these ions. Show all valence electrons and all formal charges. (a) Amide ion, NH2 (b) Bicarbonate ion, HCO3 (c) Carbonate ion, CO32 (d) Nitrate ion, NO3 (e) Formate ion, HCOO (f) Acetate ion, CH3COOarrow_forward
- The equation for the combustion of gaseous methanol is 2 CH3OH(g) + 3 O2(g) 2 CO2(g) + 4 H2O(g) (a) Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy change for this reaction. What is the enthalpy of combustion of one mole of gaseous methanol? (b) Compare your answer in part (a) with the value of tHcalculated using enthalpies of formation data.arrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. (Be sure your answers are consistent with the formal charges on the formulas.) The number of unshared pairs at atom a is со b The number of unshared pairs at atom b is H3C CH3 The number of unshared pairs at atom c is The number of unshared pairs at atom a is b H3C- The number of unshared pairs at atom b is CCH2 The number of unshared pairs at atom c isarrow_forwardBased on the atom connectivity shown bellow,evaluate the four resonance structure for the thiosulfate ion S2O3 ^2-. Use curved arrows to indicate how you get from one resonance structure to another. Assign formal changes to all atoms and determine which of these resonance structure is the most stable based on a formal charge analysis Explain your answer thoroughly. Look at the picture.arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





