
Concept explainers
(a)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and
Answer to Problem 14.33AP
The synthesis of
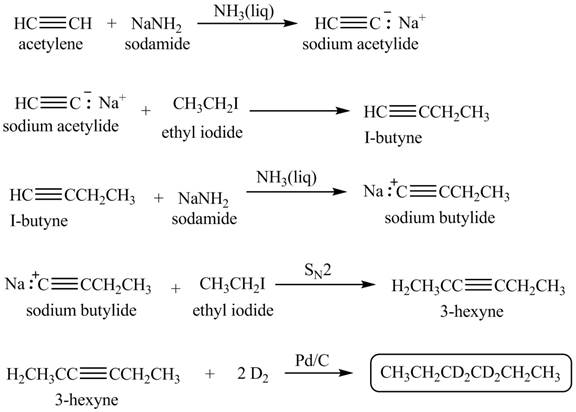
Explanation of Solution
When acetylene reacts with sodamide in
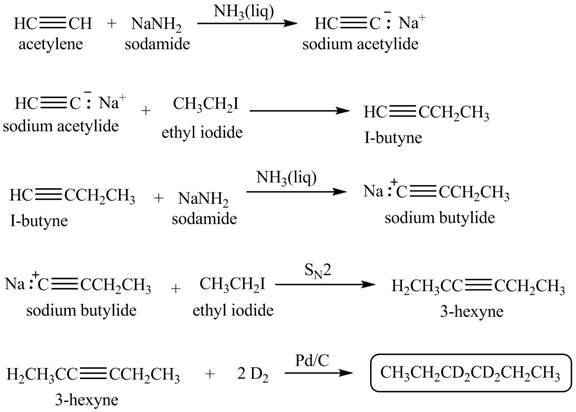
Figure 1
The synthesis of
(b)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
Explanation of Solution
When acetylene reacts with sodamide in

Figure 2
The synthesis of
(c)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
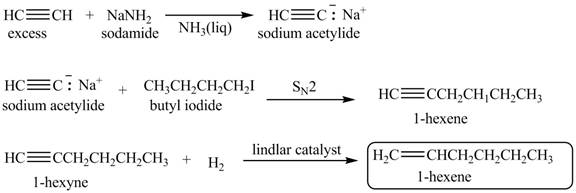
Explanation of Solution
When acetylene reacts with sodamide in
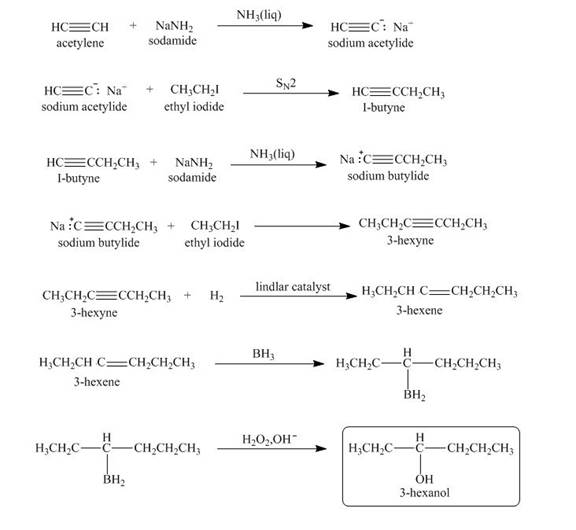
Figure 3
The synthesis of
(d)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
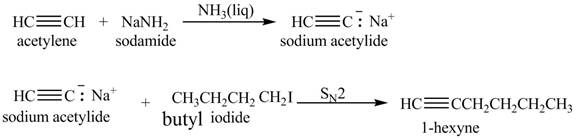
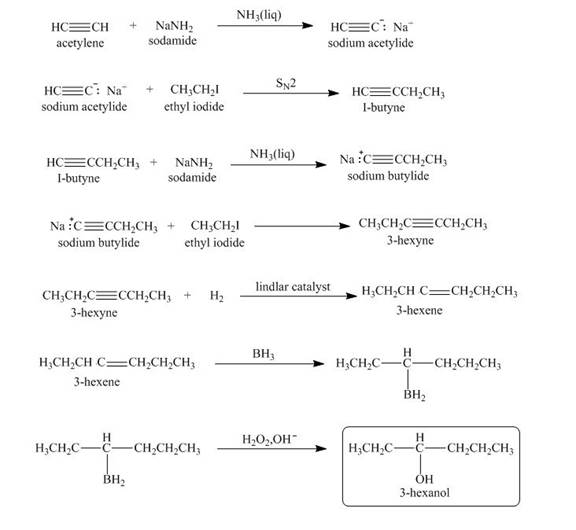
Explanation of Solution
When acetylene reacts with sodamide in

Figure 4
The synthesis of
(e)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
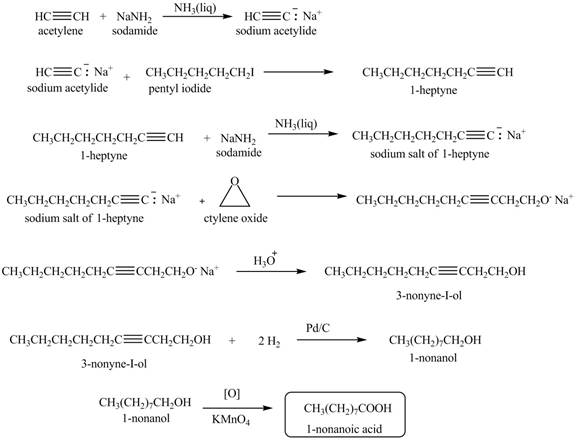
Explanation of Solution
When acetylene reacts with sodamide in
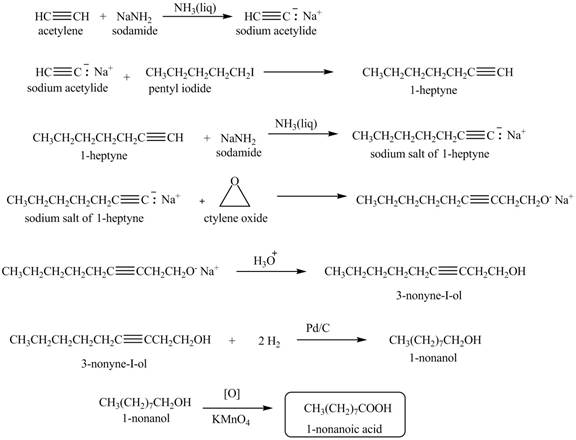
Figure 5
The synthesis of
(f)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
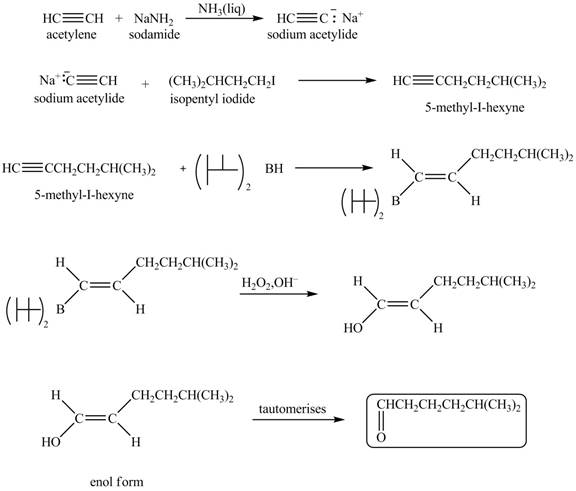
Explanation of Solution
When acetylene reacts with sodamide in

Figure 6
The synthesis of heptanal from acetylene is written as shown in Figure 6.
(g)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthsis of
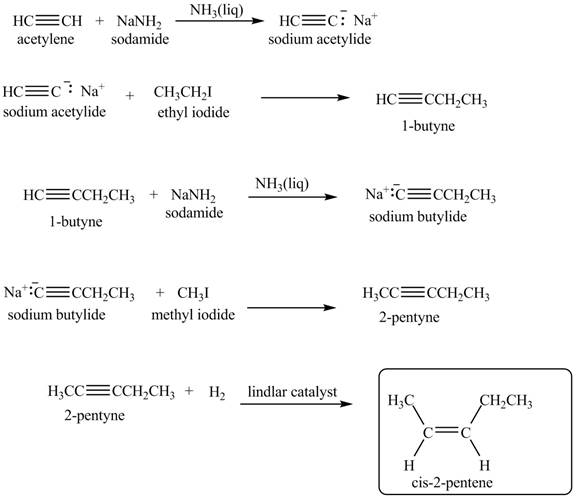
Explanation of Solution
When acetylene reacts with sodamide in

Figure 7
The synthesis of
(h)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
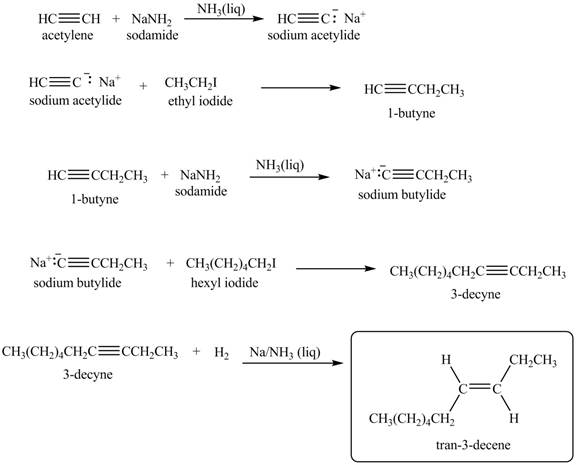
Explanation of Solution
When acetylene reacts with sodamide in

Figure 8
The synthesis of
(i)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
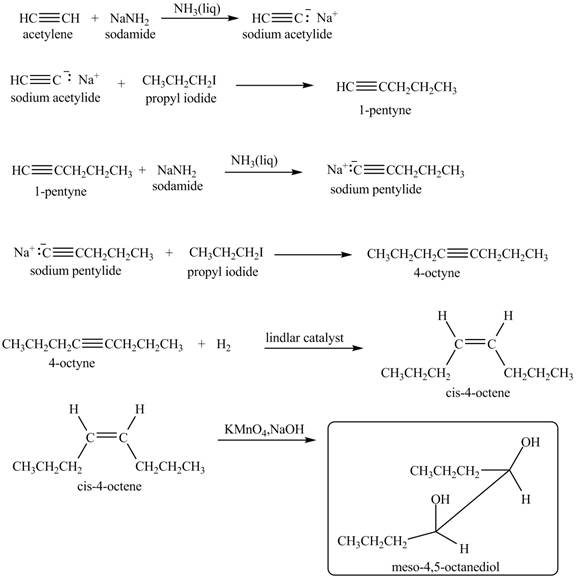
Explanation of Solution
When acetylene reacts with sodamide in

Figure 9
The synthesis of
(j)
Interpretation:
The synthesis of
Concept introduction:
There are two classes of hydrocarbon compounds, saturated and unsaturated hydrocarbons. Unsaturated hydrocarbon is of two types, alkenes and alkynes. The alkene contains a double bond between two carbon atoms. The alkynes contain a triple bond between two carbon atoms and follow a general formula
Answer to Problem 14.33AP
The synthesis of
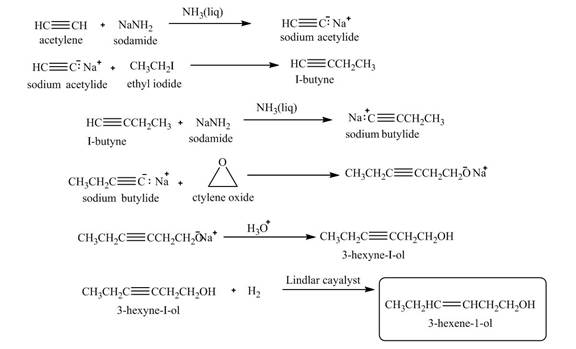
Explanation of Solution
When acetylene reacts with sodamide in

Figure 10
The synthesis of
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- Predict the major products formed when benzene reacts (just once) with the following reagents. (a) 1-chloro-2,2-dimethylpropane + AlCl3 (b) benzoyl chloride + AlCl3 (c) iodine + HNO3 (d) nitric acid + sulfuric acid(e) carbon monoxide, HCl, and AlCl3 >CuCl (f) CH2(COCl)2, AlCl3arrow_forward6) Which is the organic product for the following reaction? (a) (b) (c) (d) сон COOH ОН ОН COOH COOH KMnO4 H2Oarrow_forwardShow how you would synthesize the following compounds, starting with acetylene and any compounds containing nomore than four carbon atoms.(a) hex-1-yne (b) hex-2-yne(c) cis-hex-2-enearrow_forward
- Predict the major products (including stereochemistry) when cis-3-methylcyclohexanol reacts with the following reagents. (a) concentrated HBr (b) TsCl/pyridine, then NaBrarrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forwardprovide the structure of the intermediate and product for the following reaction : (c) H CH,OH/H (C)arrow_forward
- Write the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forwardPropose structures for molecules that fit the following descriptions:(a) An aldehyde with the formula C5H10O(b) An ester with the formula C6H12O2(c) A compound with the formula C3H7NOS that is both anamide and a thiolarrow_forward(a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forward
- Give reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forward(b) 3-methyl-2-butanol reacts with concentrated sulphuric acid to form 2-methyl-2- butene. Write the mechanism for the reaction.arrow_forwardIndicate the letter of the correct answer and kindly briefly justify the letter of answer. Cyclohexene undergoes hydrobromination. Which of these is a possible product? (A) Bromocyclohexane (B) All of these (C) Trans 1,2-dibromocyclohexane (D) Cis 1,2-dibromocyclohexanearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





