
Concept explainers
a)
Interpretation:
Number of carbon-carbon sigma bonds present in the given benzene has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
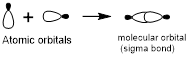
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
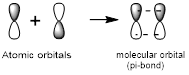
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
b)
Interpretation:
Number of carbon-carbon sigma bonds present in the given cyclobutane has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
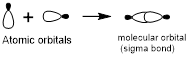
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
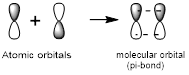
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
c)
Interpretation:
Number of carbon-carbon sigma bonds present in the given 3-ethyl-2-methylpentane has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
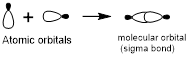
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
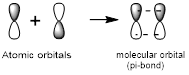
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Chemistry
- Give the IUPAC names of structures containing two carbon atoms for the following classes of compounds: (a) ether: (b) aldehyde: (c) carboxylic acid: (d) ester:arrow_forwardWhat is the molecular formula for a 5-carbon hydrocarbon with one pie bond and one ring?arrow_forwardTRUE OR FALSE (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism. (c) Cis-trans isomers have the same molecular formula but a different connectivity of their atoms. (d) Cis-2-butene and trans -2-butene can be interconverted by rotation about the carbon–carbon double bond. (e) Cis-trans isomerism is possible only among appropriately substituted alkenes. (f) Both 2-hexene and 3-hexene can exist as pairs of cis-trans isomers. (g) Cyclohexene can exist as a pair of cis-trans isomers. (h) 1-Chloropropene can exist as a pair of cis-trans isomers.arrow_forward
- write the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forwardClassify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications: alkane cycloalkane aromatic hydrocarbon alkene cycloalkene alkyne cycloalkyne CH,-C=C-CH CH, —CH, —CH, —сH (d) CHCH, (f) CCH - СНC(CH), (h) - CH2CH3 (i)arrow_forwardGive the molecular formula of a hydrocarbon containing six carbon atoms that is an aromatic hydrocarbon.arrow_forward
- The following is a structural diagram for penicillin G, an antibiotic compound with outstanding antibacterial activity. It is obtained from the liquid filtrate of molds. HO H H H C-C N- H Penicillin G can be described as an Organic Co -N- organic inorganic CH₂ H CH₂ -COOH compound. One functional group found in penicillin G is the ◆ grouparrow_forwardA certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon? (a) It is a cycloalkane. (b) It contains one ring and one double bond. (c) It contains two double bonds and no rings. (d) It is an alkyne.arrow_forwardHow many asymmetric carbons are present in Ethyl-2,2,4-tnmethypentane? Indicate them.arrow_forward
- Give the IUPAC names of structures containing two carbon atoms for the following classes of compounds: (a) alkene: (b) alkyne: (c) alkyl halide: d) alcohol:arrow_forward(a) Alkenes are relatively stable compounds but are more reactive than alkanes and serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions. Predict the products obtained from following reaction. Write the chemical reaction and name the products according to IUPAC system. (i) the reaction of 3-ethyl-3-methyl-1-pentene with hydrogen bromide (ii) the reaction of 3-ethyl-2-pentene with hydrogen bromidearrow_forwardThe IUPAC name for a carboxylic acid is based on thename of the hydrocarbon with the same number of carbonatoms. The ending -oic is appended, as in ethanoic acid,which is the IUPAC name for acetic acid. Draw the structureof the following acids: (a) methanoic acid, (b) pentanoicacid, (c) 2-chloro-3-methyldecanoic acid.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

