
Concept explainers
Expand the list of orbitais considered in Figures 5.2 and 5.3 by using all three p orbitals of atom A and all five d orbitals of atom B. Which of these have the necessary match ofsymmetry for bonding and antibondingorbitals? These combinations are rarely seen in simplemolecules but can be important in
Interpretation: The list of orbitals should be expanded using all three p-orbitals of atom A and all five d-orbitals of atom B and the orbitals should be selected which has necessary match of symmetry for bonding and antibonding orbitals.
Concept Introduction: Three conditions are considered for overlapping which leads to bonding.
- The orbital’s symmetry must be such that regions with the same sign of ψ overlap.
- The energies of atomic orbital must be comparable.
- In order to provide good overlap, the distance between the atoms should be short enough but not so short as the repulsion of other electrons or nuclei occur.
Answer to Problem 5.1P
Bonding interactions -
P orbitals and
Explanation of Solution
The shapes of p orbitals are as follows:
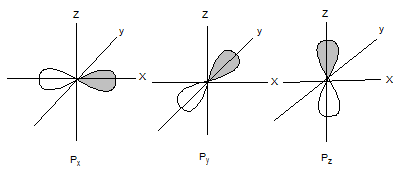
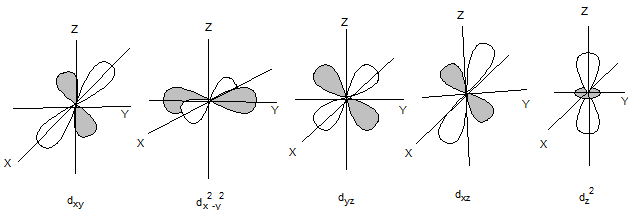
The shapes of d orbitals are as follows:
There are three possible bonding interactions. They are,
P orbitals and
Want to see more full solutions like this?
Chapter 5 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry (7th Edition)
Organic Chemistry
Organic Chemistry (9th Edition)
Chemistry For Changing Times (14th Edition)
- Compare the two complexes attached in the picture through the result of the mutual influence of π between them, explaining the reasonarrow_forwardTetrahedral complexes such as [FeF4]2- possess a Td symmetry, as indicated in the picture below. Use group theory and symmetry operations to determine the reducible representation resulting from the combination of y vectors. Break the reducible into irreducible and decide which metal orbitals are likely used to form σ bonds.arrow_forwardWrite the general electron configuration of a transition ele-ment (a) in Period 5; (b) in Period 6.arrow_forward
- 80 70 60 50 T 40 30 20 10 ATE 10 20 30 40 50 A/B O 1 and 4 2 and 2 O 3 and 3 2 and 3 3 and 4 E/Barrow_forwardWhile d-electrons in many transition metal compounds may not be involved directly in bond formation, they nevertheless exert a considerable influence on structure. Explain.arrow_forwardWhy does orbital mixing result in pale coloured transition metal complexes? Use diagrams to explain your answer.arrow_forward
- 9. Transition metals can act as Lewis bases when pi bonds on ligands are involved in bonding. Rationalize this idea and draw the effect of ligand pi orbitals can have on the LFT valence electron splitting for ML6.arrow_forwardCFT is applicable to molecules in geometries other than octahedral. In octahedral complexes, remember that the lobes of the eg set point directly at the ligands. For tetrahedral complexes, the d orbitals remain in place,but now we have only four ligands located between the axes (as given). None of the orbitals points directly at the tetrahedral ligands. However, the eg set (along the Cartesian axes) overlaps with the ligands less than does the t2g set. By analogy with the octahedral case, predict the energy diagram for the d orbitals in a tetrahedral crystal field. To avoid confusion, the octahedral eg set becomes a tetrahedral e set, and the octahedral t2g set becomes a t2 set.arrow_forwardLet’s consider octahedral Sc3+ and Ti3+ complexes. How many d electrons will each have? Sc3+: 0; Ti3+: 0 Sc3+: 0; Ti3+: 1 Sc3+: 1; Ti3+: 0 Sc3+: 1; Ti3+: 1arrow_forward
- The two egorbitals are identical in energy in an octahedralcomplex but have different energies in a square planar complex,with the dz2orbital being much lower in energy than the dx2-y2 .Explain with orbital sketches.arrow_forwardAccording to valence bond theory, what set of orbitals isused by a Period 4 metal ion in forming (a) a square planarcomplex; (b) a tetrahedral complex?arrow_forwardthe hybridization is (Zn, Z-30). The structure of the complex [ZnCla" is Tetrahedral, diamagnetic, spa O Square planar, paramagnetic, dsp.b Tetrahedral, paramagnetic, sp.c Square planar, diamagnetic, dsp darrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





