
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.14B, Problem 7.27P
Make models of the blowing compounds, and predict the products formed when they react with the strong bases shown.
a. 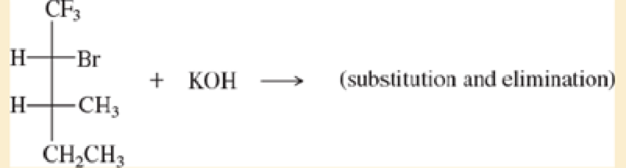
b. meso-1,2-dibromo-1,2-diphenylethane+(CH3CH2)3N :
c. (d,l)-l,2-(dibromo-1,2-diphenylethane+(CH3CH2)3N :
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the compound in each pair is more acidic?
a. (i) 2,2-dichloropropan-1-ol or (ii) 2,2-difluoropropan-1-ol
b. (i) cyclopentanol or (ii) 3-chlorophenol
c. (i) cyclohexanol or (ii) cyclohexanecarboxylic acid
1. Which compound has the lowest boiling point?A. 2,6-Dibromophenol
B. Hexan-2-one
C. Octan-1-olD. 1-Chlorohexane
2. Arrange following compounds in decreasing order of their acid strength
2,4,6-trinitrophenol
3,5-dinitrophenol
3-nitrophenol
phenol
4-methvlphenol
Propan-1-ol
3. Which of the following compounds are incapable of hydrogen bonding?A. AlcoholB. None ol the choicesC. PhenolD. Ether
4. Which of the following is the basis of the classification of alcohols?A. Based on the number of hydroxyl groups altachcoB. Based on the number of carbon atoms which are directly attached to the carbon that is bonded with the -OH groutC. BothD. Neither
a. Which is a stronger acid? a or b?
NH2
NH3
a
b
b. If nitration is done on both of them, draw the structures of the products?
c. Identify a,b,c, and d
CH;CH2CHO
OzJZn/CH3COOH
b+ C
Ether
d.
Li(CH3)2Cu/H30*
T T T
Arial
A 5 (18p + T
Path: p
Words:0
QUESTION 12
a. How many different proton signals and carbon signals will be observed for the following compound in its proton
NMR spectrum? Indicate the proton spin multiplicity?
H3C
CH-O-C-CH2-CH
b. Predict the products of the reaction with acetaldehyde with the following reagents?
a. NABH4, then H30*
b. Dess_Martin reagent
c. Methylmagnesium bromide, then H30*
d. (C6H5)3P=CH2
Chapter 7 Solutions
Organic Chemistry (9th Edition)
Ch. 7.3A - Prob. 7.1PCh. 7.3A - Prob. 7.2PCh. 7.3B - Draw five more compounds of formula C4H6NOC1.Ch. 7.3B - For each of the following molecular formulas,...Ch. 7.4 - Give the systematic (IUPAC) names of the following...Ch. 7.5B - The following names are all incorrect. Draw the...Ch. 7.5B - Prob. 7.8PCh. 7.5B - a. How many stereogcmc double bonds are in...Ch. 7.6 - Teflon-coated frying pans routinely endure...Ch. 7.7B - Prob. 7.11P
Ch. 7.8B - Use the data in Table7-2 to predict the energy...Ch. 7.8C - Prob. 7.13PCh. 7.8E - Explain why each of the following alkenes is...Ch. 7.8F - Prob. 7.15PCh. 7.10 - Prob. 7.16PCh. 7.10A - SN1 substitution and E1 elimination frequently...Ch. 7.10C - Prob. 7.18PCh. 7.10C - Prob. 7.19PCh. 7.10C - Prob. 7.20PCh. 7.11 - Prob. 7.21PCh. 7.11 - Prob. 7.22PCh. 7.12 - Prob. 7.23PCh. 7.12 - Prob. 7.24PCh. 7.13 - Prob. 7.25PCh. 7.14B - Prob. 7.26PCh. 7.14B - Make models of the blowing compounds, and predict...Ch. 7.15 - Prob. 7.28PCh. 7.15 - Prob. 7.29PCh. 7.15 - Prob. 7.30PCh. 7.15 - Prob. 7.31PCh. 7.16 - Predict the major and minor elimination products...Ch. 7.17B - Predict the products and mechanisms of the...Ch. 7.18 - Propose mechanisms for the following reactions.Ch. 7.18 - Prob. 7.35PCh. 7.19B - The dehydrogenation of butane to trans-but-2-ene...Ch. 7.19B - Prob. 7.37PCh. 7.19B - Prob. 7.38PCh. 7.19B - Prob. 7.39PCh. 7 - Prob. 7.40SPCh. 7 - Prob. 7.41SPCh. 7 - Prob. 7.42SPCh. 7 - Prob. 7.43SPCh. 7 - Prob. 7.44SPCh. 7 - Prob. 7.45SPCh. 7 - Prob. 7.46SPCh. 7 - The energy difference between cis- and...Ch. 7 - Prob. 7.48SPCh. 7 - Prob. 7.49SPCh. 7 - Prob. 7.50SPCh. 7 - What halides would undergo E2 dehydrohalogenation...Ch. 7 - Prob. 7.52SPCh. 7 - Prob. 7.53SPCh. 7 - Write a balanced equation for each reaction,...Ch. 7 - Prob. 7.55SPCh. 7 - Using cyclohexane as your starting material, show...Ch. 7 - Show how you would prepare cyclopentene from each...Ch. 7 - Prob. 7.58SPCh. 7 - E1 eliminations of alkyl halides are rarely useful...Ch. 7 - Prob. 7.60SPCh. 7 - Propose mechanisms for the following reactions....Ch. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Prob. 7.64SPCh. 7 - Prob. 7.65SPCh. 7 - Prob. 7.66SPCh. 7 - Prob. 7.67SPCh. 7 - Prob. 7.68SPCh. 7 - Prob. 7.69SPCh. 7 - Explain the dramatic difference in rotational...Ch. 7 - One of the following dichloronorbornanes undergoes...Ch. 7 - A graduate student wanted to make...Ch. 7 - Prob. 7.73SPCh. 7 - Prob. 7.74SPCh. 7 - Prob. 7.75SPCh. 7 - Prob. 7.76SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Give one example from main group chemistry that illustrates each of the following descriptions: (a) Covalent ne...
General Chemistry: Atoms First
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- i. h. g. f. CH3CH2CH2NH2 + HCI CH3CH2- OH + CH3 CH—NH, CH3 CH3CH2CH2CH2-C=N H2(xsy/Pt NH3+ CH3-CH-CH2-CH₂CH₂ Base CIarrow_forward1. Give the products of the acid-base reactions below and indicate whether products or reagents would be favored a. CH3CO2 CF3CH2OH t b. H3N CH30 Na* H2N NH3 C. НАР* d. H3N H4C CH3 C C е. f. H30 NH3 CH3CO2H g. H20 h. H20 H2S 2. Draw the most stable conjugate acid for each of the three heterocycles below. Of these three nitrogen heterocycles, which is the least basic? Which is the most basic? H Н H H N H нн H 3. Which atoms in the following structures have the greatest partial negative charge Нас Нзс OCH2CH2CCF2CF2CF20 HNO NH2 NH . 4. Which of the following is most acidic? D C B A "C(CH)з (H3C)3C CH3 Hас N. CF3CF3 H3C CH3 Hас" "CНз z. I CD :Z I Z-Iarrow_forward(c) Using the reagents below, list in order (by letter, no period) those necessary to prepare compound C from Note: Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. Br₂ b. HBr c. HCN d. NBS, light e. H₂O, H₂SO4 f. NaCN g. NaOEt, EtOH Step #1 Step #2 e Step #3 naarrow_forward
- Give the structure of compounds A to Fin the following series of reactions: CH,0 CH-CH-C-a A HNO, Fe, HCI B. AICI H,SO, Pd/H2 Brz. FeBr KMNO, D.arrow_forward10. E to CH3ONa a) Br₂ Br2 1. Mg /ether khan (A) (B) (C) (D) hv FeBr3 hv 2. CO2 3. H3O+ C1 B2 B3 B1 KMnO4 H+ C1 Mg, ether CHO b) 1. CH3Mgl H2SO4 HBr 1. Mgether khan A B D to 2. H3O+ peroxide 2. H₂C-CH2 ° CH3COCI AICI 3 1. C1 1. 03 G F E 2. H3O+ 2. Na₂S 3. H3O+arrow_forwardWhich product will be formed from this E2 reaction ? CH2C H3 H CHCH3 H CH2CH3 CH2CH, base (E2) H2C CH2CH3 H. H;C H. H. H,C H3C H3C H. II II IV O a. IV only Ob. II and IV O c. II and IV Od.lonly O e. Il onlyarrow_forward
- مگر compound e Reagents a. HX b. c. H₂O, H₂SO4 d. X₂ e. H₂. Pd 1. X₂, H₂O g. Oso, then NaHSO, h. 1. j. HBr, H₂O₂. hv k. 1. compound a bb Hg(OAc)₂, H₂O then NaBH, BH, then H₂O₂. NaOH O, then (CH₂)₂S 2 equivalents of NaNH₂ H₂, Lindlar's catalyst P. adi✔ m. Na/NH₂ n. H₂SO₂, HgSO4 o 9₁ bb r. compound b compound c S L PBr u SOCI₂ V. H₂PO w. H₂CrO₂ X PCC compound f (sia) BH then H₂O₂, NaOH 1 equivalent of NaNH₂ NBS, hy Br₂, hv (CH₂)₂CO¹K* compound d y. Z aa. bb. Na 104 mCPBA NaOH, H₂O compound garrow_forwardCH;CH,Mgl, then aq. acid workup 1. Mg, anhydrous ether 2. Н-о -Br g. OCH3 H3C-B OCH, Pd catalyst Br h. H,C MgBr (excess) OCH3 then aq. acid workup j. Pd catalyst Heck Reactionarrow_forward7. Draw all resonance structures for the following species: a. C6H5CH₂ b. c. CH3CH=CHCH=CHCH=CHCH₂ C. 8. (S)-2-Butanol slowly racemizes on standing in dilute sulfuric acid. Explain with mechanism.arrow_forward
- Identify the reagents you would use to achieve the following transformations: Reagents 1 Reagents 2 Select from the given reagents: A. Na, Pt B. H2, NH3 (1) C. H2, Lindlar's cat. D. Na, NH3 (I) E. H2, Pt F. Na, Lindlar's cat. Reagents 1 and Reagents 2arrow_forwardBr ÓCH, a. SN1 & E1 b. SN1 & E2 c. SN2 & E1 d. SN2 & E2 21. What is the reagent used for the following reaction? foid W a. BH3/THF, then H2O2/OH b. Hg(OAc)2/H2O, then NaBH4 с. Н2О, Н* d. H2, Pd/C 22. What is the major product for reaction below? H,SO4 OH d. none b. с. a. 3. Arrange the following substrates in order of increasing their SN2 reactivity with NaC bentane,arrow_forwardPredict the correct product from the reaction shown. (1) Hg(OAc); THF, HO (1) (2) NaBH, HO (2) HO HO Compound (3) a. b. Compound (1) Compound (4) C. Compound (2) d.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY