
Concept explainers
a) -CH3, -OH, -H, -Cl
Interpretation:
The substituents in the set, -CH3, -OH, -H, -Cl, are to be ranked according to the sequence rules.
Concept introduction:
The member that ranks higher can be determined by considering the
To rank:
The substituents in the set, -CH3, -OH, -H, -Cl, according to the sequence rules.
b) -CH3, -CH2CH3, -CH=CH2, -CH2OH
Interpretation:
The substituents in the set, -CH3, -CH2CH3, -CH=CH2, -CH2OH, are to be ranked according to the sequence rules.
Concept introduction:
The member that ranks higher can be determined by considering the atomic number of the first atom in each substituent. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms can be considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms.
To rank:
The substituents in the set, -CH3, -CH2CH3, -CH=CH2, -CH2OH, according to the sequence rules.
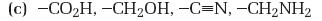
Interpretation:
The substituents in the given set are to be ranked according to the sequence rules.
Concept introduction:
The member that ranks higher can be determined by considering the atomic number of the first atom in each substituent. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms can be considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms.
To rank:
The substituents in the given set according to the sequence rules.
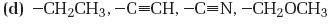
Interpretation:
The substituents in the given set are to be ranked according to the sequence rules.
Concept introduction:
The member that ranks higher can be determined by considering the atomic number of the first atom in each substituent. The atom with highest atomic number gets the higher rank. If a decision cannot be made by considering the atomic number of the first atom in each substituent then the second, third, fourth atoms can be considered until the first difference is found. Multiple bonded atoms are considered as equivalent to the same number of single bonded atoms.
To rank:
The substituents in the given set according to the sequence rules.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry
- Name the following hydrocarbons: H;C CH3 (a) H,C=Ċ-Ċ=CH, H H (b) H;C-C=C-C=C-CH3 H H CH3 (c) H;C-C-CH,–CH3 CH3 CH, (d) H;C-C-CH3arrow_forwardDraw both cis- and trans-1,4-dimethylcyclohexane in their more stable chairconformations. (a) How many stereoisomers are there of cis-1,4-dimethylcyclohexane, and how many of trans-1,4-dimethylcyclohexane? (b) Are any of the structures chiral? (c) What are the stereochemical relationships among the various stereoisomers of 1,4-dimethylcyclohexane?arrow_forward(a) Draw all stereoisomers formed by monobromination of the cis and trans isomers of 1,2-dimethylcyclohexane drawn below. (b) How do the products formed from each reactant compare-identical compounds, stereoisomers, or constitutional isomers? cis-1,2-dimethylcyclohexane trans-(1R.2S)-dimethylcyclohexanearrow_forward
- CH3 H3C N- -CH2 H2C CH2 H3C (1) CH3 CH3 CH3 H2 Br CH3 CH2 ČH3 (2) CH HC CH CHarrow_forwardWrite down the IUPAC name and structure of the followings (i) HOH,C-CH = CH - CH,OH (ii) H.C =C-CH,-CH,-OH CH, (iii) HC = C-CH,-CH,-CH-CHO (iv) CH,COCOOH (v) CH,-CH-CHO OH ČCI (vi) CH,-CH- -ċ-CH-OCH, CH, CH, CH-CCI, (viii) CH CH = CH - COOH (vii) CH-CCI, (ix) CH,-CH-C-CH-OCH,CH, ÓCH, CH, (x) CH,-CH-CH, ОН Он ОН (xi) 2,4-dichlorohexane (xii) 6-methylhept-3-enal (xiii) 3-methylpentane-2,4-dione (xiv) 2-bromo-5-methylhexanoic acid (xv) 3-methylpentan-3-olarrow_forwardD. Rank (increasing) the substituents in each of the following sets: (а) -Н, -ОН, -ОСH3, -СH, (Б) -Вr, -СНз, -СНBr, -а (c) -CH=CH2, -CH(CH3)2, -C(CH3)3, -CH 2CH3arrow_forward
- (a) (b) 1. CH3MgBr 2. H3O+ H3C OH I H3C-C-C-CH3 H3C H ОН H CH3 CH3 H3C CH3 H3C H₂SO4 C=Carrow_forward-C-Br+ HD H2O 250 (cH,CH>),C-Br (CH3CH2), -C- Br 750arrow_forwardmethyl-CH3 -CH,CH3 propyl -CH,CH,CH3 butyl ethyl -CH,CH,CH,CH, pentyl -CH,CH,CH,CH,CH, hexyl -CH,CH,CH,CH,CH,CH, heptyl -CH,CH,CH,CH,CH,CH,CH, octyl -CHSCH,CH,CH,CH,CH,CH,CH nonyl -CH,CH,CH,CH,CH,CH,CH,CH, decyl -CH,CH,CH,CH,CH,CH CH,CH, Give the name for this molecule %3D CH, = CH-Ĉ-O-Harrow_forward
- CH2 CH2 CH2 CH2 CH2 CH2 CH3 HO-CH-CH=CH CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 CH-NH-C-CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 CH2-O-P-0-CH2-CH2-N-CH3 CHa CH3 Download Image... Based on the backbone, to which lipid class does the lipid belong? O A. Derived lipid O B. Glycerophospholipid OC. Triacylglycerol O D.Sphingolipidarrow_forward(A)Menthol, used to flavor various foods and tobacco, is the most stable stereoisomer of 2-isopropyl-5-methylcyclohexanol. Draw its most stable conformation. Is the hydroxyl group cis or trans to the isopropyl group? To the methyl group? (b) Neomenthol is a stereoisomer of menthol. That is, it has the same constitution but differs in the arrangement of its atoms in space. Neomenthol is the second most stable stereoisomer of 2-isopropyl-5methylcyclohexanol; it is less stable than menthol but more stable than any other stereoisomer. Write the structure of neomenthol in its most stable conformation.arrow_forward• How do you differentiate the following pair of compounds by IR? H, H2 H H,c-Cc-CH, H2 1 H;C CH3 H2 H2 -CH3 H2 2 H,C H2 H2 H,c-Cc-CH, H2 H,c-CCECH H;C 4arrow_forward
