
(a)
Interpretation:
The numbers of chlorination products obtained from radical chlorination of methyl cyclohexane has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
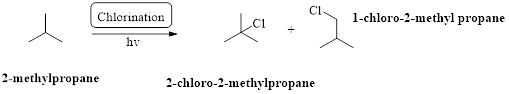
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
The mechanism of monochlorination of ethane (as an example) includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,
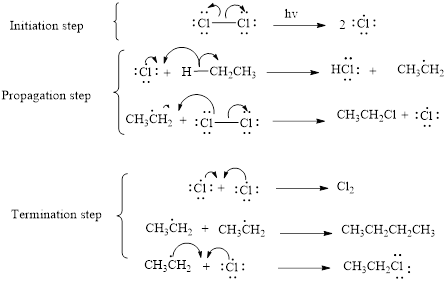
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat
(b)
Interpretation:
The number of monochlorination products obtained from the radical chlorination of methyl cyclohexane should be given when all stereoisomers are included.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
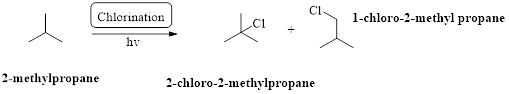
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
The mechanism of monochlorination of ethane (as an example) includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,

In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called enantiomers
Total number of stereoisomers = 2n
Where n is the number of chiral centers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry (3rd Edition)
- Why Radical Chlorination 2,2-dimethylpropane yield only single product? Give the name of the product.arrow_forwardThe synthesis of cyclohexanone oxime from cyclohexanone is a net? a. elimination b. substitution C. addition d. rearrangement The synthesis of cyclohexanone oxime from cyclohexanone is a net of carbon? a. reduction b. oxidation C. not a redox of carbon Balancing the equation of all the starting reagents going to final products in the synthesis of cyclohexanone oxime from cyclohexanone (see the first question), what is the coefficient of water if the coefficient of cyclohexanone is one? Give your answer in decimal form with no units. Answer: Select all the effects that you should see in an infrared going from cyclohexanone to cyclohexanone oxime. Selecting wrong answers may give you a negative score. a. a peak should disappear from above 3000 cm -1 b. a new peak should appear above 3000 cm -1 c. the frequency of the infrared absorption should go down in the double bond region d. the frequency of the infrared absorption should go up in the double bond regionarrow_forwardHow many moles of Bra are required to completely halogenate the alkene?A. One moleB. Two molesC. Three molesD. Four moles What is the expected arrangement of the bromine atoms relative to each other amongthe carbon involved in pi bonding?A. anti-conformationB. syn-conformationC. trans-configurationD. cis-configuration What happens to bromine when it is adjacent to an alkene during a chemical reaction?A. Bromine becomes stable. (? kasi before brown siya/acidic tas naging colorless? Jk ewan)B. Bromine becomes polarized.C. Bromine becomes hybridized.D. Bromine becomes acidic. The relative arrangement of bromine atoms in the product is primarily due to:A. ElectronegativityB. RepulsionC. Hydrogen bondingD. Atomic weightWhat is your observation after the reaction?A. A yellow flame is produced.B. Bromine water decolorizes.C. The alkene becomes denser.D. A brown precipitate forms.arrow_forward
- Radicals and carbocations are electrophiles. Define how and why ?arrow_forwardMonobromination Reaction. Draw the products for the monobromination reaction of 1,2-dimethylcyclopentane. Calculate the relative percentages of the products and determine the major productarrow_forwardExplain Chlorination Versus Bromination ?arrow_forward
- a) Give the molecular formula and draw the skeletal structure for 3-bromo-3- methylhexane. b) Name (including E/Z stereochemistry) the FIVE alkenes that can produce 3-bromo-3- methylhexane on reaction with HBr. Draw the skeletal structure of each molecule.??? c) Define the type of stereoisomerism present in 3-bromo-3-methylhexane. Name and draw the tetrahedral representation of the two stereoisomers?. d) For the base-catalysed hydrolysis of 3-bromo-3-methylhexane (i.e. reaction with the nucleophile OH-): i) ii) iii) iv) State whether the reaction is likely to proceed by an SN1 or SN2 mechanism, and explain your choice Give the likely rate law for the reaction. Explain your choice. For the reaction intermediate, draw its structure and give the VSEPR description of the geometry at the reaction centre Give the names and draw the structures of the two reaction products. Explain your conclusionsarrow_forwardfluorination of alkanes is highly exothermic. Per Hammond’s postulate, assume that the transition state for radical fluorination is almost identical to the starting material. Assuming this fact, estimate the fraction of each monofluoro product formed in the fluorination of 2-methylbutane.arrow_forwardWrite the monobromination products of 1,4-dimethylcyclohexane and calculate the percentages of each product. Which is the major product?arrow_forward
- 2. In the bromination reactions, what is the function of CCl4? Why can it fulfil its role?3. Bromination proceeds by either free radical substitution or electrophillic addition. Based on Table 3, which mechanism is followed by alkanes? by alkenes? by alkynes?4. For which hydrocarbon type is light necessary for bromination to take place?5. What is the function of light in the bromination reaction? Why are alkenes and alkynes not included as samples?arrow_forward10. In free-radical substitution reaction of alkanes with halogens under uv light, A. the photolytic breaking of the halogen is the rate determining step. B. the abstraction of hydrogen from alkane by the halogen radical is the rate determining step C. the formation of alkylradical is the rate determining step. D. the formation of halogen radical is the rate determining step. 11. Which of the following processes could be the termination step in free radical substitution reaction? A. C₂H6 ---→ 2CH3- B. C₂H6 +H --⇒ H₂ + C₂H5 C. CH3 + CH3 --→ C₂H6 D. C₂H5 --→ C₂H4 + Harrow_forwardAlkane chlorination can occur at any position in the alkane chain. Draw and name all monochloro products you might obtain from radical chlorination of 3-methylpentane.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





