
Interpretation:
The number of monochlorination products obtained from the radical chlorination of 2-methylbutane including all stereoisomers should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
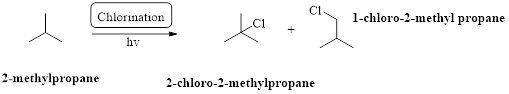
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called enantiomers
Total number of stereoisomers = 2n
Where n is the number of chiral centers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry (3rd Edition)
- Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are percent in cyclopropane? What fraction of the overall 115 kJ/mol (27.5 kcal/mol) strain energy of cyclopropane is due to torsional strain.arrow_forward08.20b 1) ВН, THF 2) H,O2, NaOH Modify the given carbon skeleton to draw the major product(s) of the given reaction. If a racemic mixture of enantiomers is expected, draw both enantiomers. Note: you can select a structure and use Copy and Paste to save drawing time. CHarrow_forwardDraw the organic product that is expected to form when the following compound is oxidized under biological conditions. oxidation reduction 0 SH • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. If no reaction occurs, draw the organic starting material. / BO O-Sn [F ChemDoodle Previous Marrow_forward
- Draw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. ● CEC • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu. MULL ? H₂O/H₂SO4/HgO ChemDoodleⓇ Sn [Farrow_forwardGive IUPAC names for the following structures. When appropriate, abbreviate ortho (o), meta (m), and para (p), no italics, if you elect to use these terms. CH₂CH₂CHCHCHSCHCH₂ I T CH₁ CH₂ 1st structure: CH3 CH3 I 2nd structure: Submit Submit Answer Try Another Version OCH3 CH₂CCH₂ OCH3 10 item attempts remainingarrow_forwardDraw a structural formula for the substitution product of the reaction shown below. Br Na ОCH3 H3C CH3OH • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu. • Products that are initially formed as ions should be drawn in their neutral forms. C opy aste ChemDoodle'arrow_forward
- OCCH, [Review Topics] [References] Draw a structural formula for the substitution product of the reaction shown below. F + - CH3 OCCH3 Na CH;CO,H • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu. n [F ChemDoodle Cengage Learning | Cengage Technical Support MacBook Air 80 000 DII DD F4 F5 F6 E7 F8 F10arrow_forwardCH₂CH=CHCHCH3 CH3 + VIL • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • If there is more than one major product possible, draw all of them. Separate multiple products using the + sign from the drop-down menu. CH4 > / ? HBr ChemDoodle ? Sn [Farrow_forwardReview Topics] [References] Draw a structural formula for the major product of the reaction shown. CH;CH2 c=CHCH3 CH;CH2 Br2 H20 • Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. • Do not show stereochemistry in other cases. • If the reaction produces a racemic mixture, just draw one stereoisomer. Previous Next ChemDoodle Save and Ex tv ill I 11 МacBook Air DII DD F12 F11 F10 80 888 F9 F7 FB F6 F5 F4 F3 * & $ %3D 5 6. 7 8 9. 4 { P E R T Y H J K F + * COarrow_forward
- Draw a structural formula for the substitution product of the reaction shown below. H3C H Br Na' acetone • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. Separate multiple products using the + sign from the drop-down menu. • Products that are initially formed as ions should be drawn in their neutral forms. C P opy aste [F ChemDoodlearrow_forwardDraw the product that would form when 4-methyl-2-pentene reacts with bromine. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. / Sn [F ? ChemDoodlearrow_forward3. BIn من لب Classify steps I, II, and III as substitution, elimination, or addition reactions (J. Org. Chem., 2011, 76, 3968) BI no , -CH3 'N' CH3 N I -CH3 KOH من سي سي CH3 I KOH II OH , -CH3 N CH3 CH; N CH3 III OH 'N ا -CH3 CH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

