
Concept explainers
17-73 Alcohols can be prepared by the acid-catalyzed hydration of
(a) Ethanol
(b) Cyclohexanol
(c) 2-Propanol
(d) 1-Phenylethanol
(a)
Interpretation:
Show the preparation of ethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
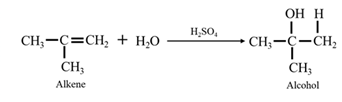
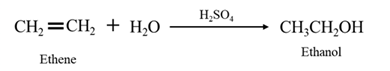
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
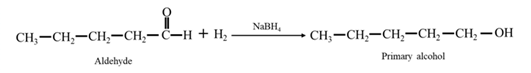
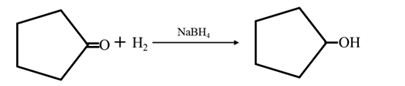
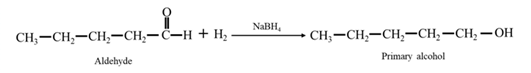
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 17.73P
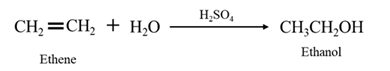
By acid-catalyzed hydration of ethane:
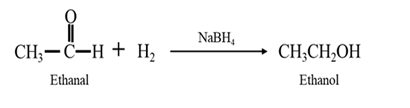
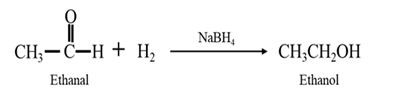
By Reduction of ethanal:
Explanation of Solution
By acid-catalyzed hydration of ethane:
When ethene is allowed to react with water in presence of an acid catalyst it gives ethanol.
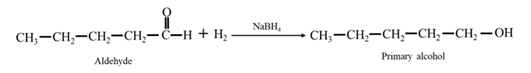
By Reduction of ethanal: When ethanal is reduced in the presence of sodium borohydride it gives ethanol.
(b)
Interpretation:
Show the preparation of cyclohexanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
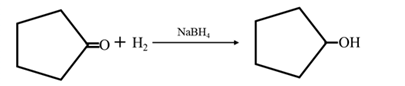
Answer to Problem 17.73P
By acid-catalyzed hydration of ethane:
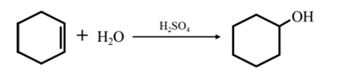
By Reduction of ethanal:
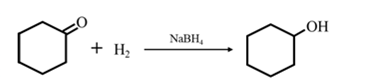
Explanation of Solution
By acid-catalyzed hydration of ethane: When cyclohexene is allowed to react with water in presence of an acid catalyst it gives cyclohexanol.
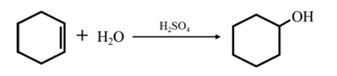
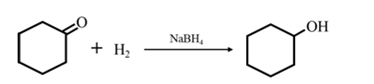
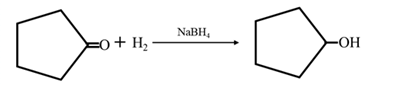
By Reduction of ethanal: When cyclohexanone is reduced in the presence of sodium borohydride it gives cyclohexanol.
(c)
Interpretation:
Show the preparation of 2-propanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
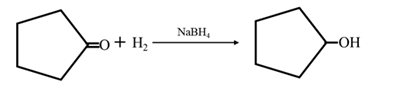
Answer to Problem 17.73P
By acid-catalyzed hydration of ethane:
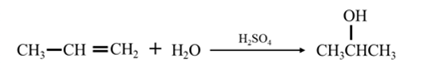
By Reduction of ethanal:
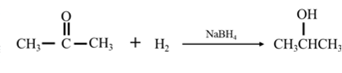
Explanation of Solution
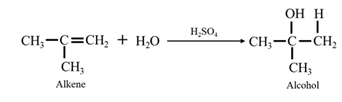
By acid-catalyzed hydration of ethane: When propene is allowed to react with water in presence of an acid catalyst it gives 2-propanol.
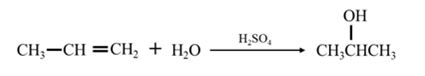
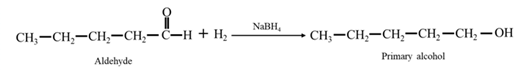
By Reduction of ethanal: When acetone is reduced in the presence of sodium borohydride it gives 2-propanol.
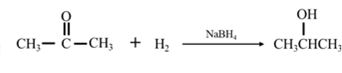
(d)
Interpretation:
Show the preparation of 1-phenylethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 17.73P
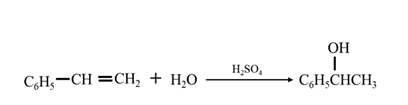
By acid-catalyzed hydration of ethane:
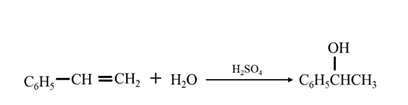
By Reduction of ethanal:
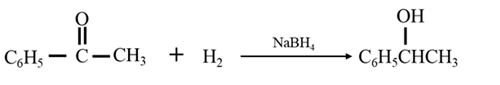
Explanation of Solution
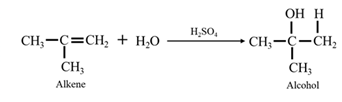
By acid-catalyzed hydration of ethane: When 1-phenylethene is allowed to react with water in presence of an acid catalyst it gives 1-phenylethanol.
By Reduction of ethanal: When acetophenone is reduced in the presence of sodium borohydride it gives 1-phenylethanol.
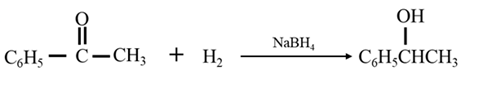
Want to see more full solutions like this?
Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
- 17-60 1-Propanol can be prepared by the reduction of an aldehyde, but it cannot be prepared by the acid catalyzed hydration of an alkene. Explain why it cannot be prepared from an alkene.arrow_forward17-3 1 Draw a structural formula for the principal organic product formed when each compound is treated with K2Cr2O7/H2SO4. If there is no reaction, say so. (a) Butanal (b) Benzaldehyde (c) Cyclohexanone (d) Cyclohexanolarrow_forwardDraw the structural formula of an alkene that undergoes acid-catalyzed hydration to give each of the following alcohols as the major product. More than one alkene may give each compound as the major productarrow_forward
- 17-70 What simple chemical test could you use to distinguish between the members of each pair of com pounds? Tell what you would do, what you would expect to observe, and how you would interpret your experimental observation. (a) Benzaldehyde and cyclohexanone (b) Acetaldehyde and acetonearrow_forward14-78 Consider alkenes A, B, and C. each of which has the same molecular formula, C(.H12. Alkenes B and C can each be separated into cis and trans isomers. Upon catalytic reduction using H,, in the presence of a transition metal catalyst (Ni, Pd, or Pt>, alkenes A, B, and C all give hexane as the only product. Acid- catalyzed hydration of alkene C gives one alcohol with the molecular formula CeH14O. Acid catalyzed- hydration of alkene B gives an equal mixture of two alcohols, each with the molecular formula C6H14O. Acid-catalyzed hydration of alkene C gives only a single alcohol with the molecular formula C6H14O. Propose structural formulas for alkenes A, B, and C and the alcohols formed by acid-catalyzed hydration of each, consistent with these experimental results.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forward
- A solid sticky substance which strongly repels water is made of the following molecules: CH3 (CH2)5 — || (CH2)14-CH3 If it is treated with sulfuric acid and heat, two new substances can be recovered. One is an alcohol. Write the chemical formula of the alcohol. Give the common (not IUPAC) name of the other substance. 0arrow_forwardDescribe each highlighted bond in terms of the overlap of atomic orbitals. and Draw the structures of ALL of the aldehydes with the molecular formula C5H10O that contain a 5-carbon chain.arrow_forwardAlcohols undergo dehydration reactions in the presence of an acid catalyst. Which of the following compounds yields only a single alkene product upon dehydration?arrow_forward
- Rank the following alcohols in order of increasing ease of acid-catalyzed dehydration. Provide the structure of the dehydration product (alkene) from each alcohol. OH OH 3 1 a OHarrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H100) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCI) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardThree constitutional isomers of molecular formula C 5H 8O can be converted to 1-pentanol (CH 3CH 2CH 2CH 2CH 2OH) on treatment with two equivalents of H 2 in the presence of a Pd catalyst. Draw the structures of the three possible compounds, all of which contain a carbonyl grouparrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
