
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.11P
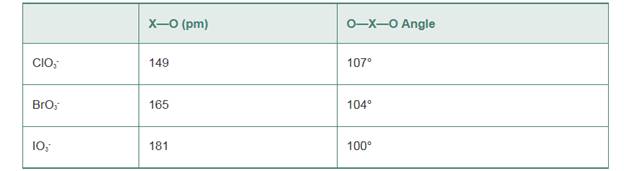
Explain the trends in bond angles and bond lengths of the following ions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(b) Arrange the following in decreasing order of bond length and bond angle with
explanation
C2H6, C2H4, C¿H2
Your assigneed ion is : AlCl4- please give a brief description that includes:
(f) actual bond angles (may use > or< symbols where appropriate).
Please note that your individual assignment may be for an ion (don't forget about charges) or a neutral atom.
Arrange the following in decreasing order of bond length and bond angle with explanation C2H6, C2H4, C2H2
Chapter 3 Solutions
Inorganic Chemistry
Ch. 3.1 - Use electron-dot diagrams and formal charges to...Ch. 3.2 - Predict the structures of the following ions....Ch. 3.2 - Prob. 3.3ECh. 3.2 - Which molecule has the smallest bond angle in each...Ch. 3.2 - Briefly account for the following observations: a....Ch. 3.2 - Does this approach work for different steric...Ch. 3 - The dimethyldithiocarbamate ion, [S2CN( CH 3 )2]-...Ch. 3 - Several resonance structures are possible for each...Ch. 3 - Draw the resonance structures for the...Ch. 3 - Three isomers having the formula N2CO are known:...
Ch. 3 - Show the possible resonance structures for nitrous...Ch. 3 - Nitric acid, which exists as HNO3 molecules in the...Ch. 3 - L. C. Allen has suggested that a more meaningful...Ch. 3 - Give Lewis dot structures and sketch the shapes of...Ch. 3 - Give Lewis dot structures and sketch the shapes of...Ch. 3 - Give Lewis dot structures and sketch the shapes of...Ch. 3 - Explain the trends in bond angles and bond lengths...Ch. 3 - Select from each set the molecule or ¡on having...Ch. 3 - a. Compare the structures of the azide ion, N3 ,...Ch. 3 - Consider the series OCl2,O( CH3)2 , and O( SiH3)2...Ch. 3 - Two ions isoelectronic with carbon suboxide, C3O2...Ch. 3 - Explain the following: a. Ethylene, C2H4 , is a...Ch. 3 - Explain the following: a. PCI5 is a stable...Ch. 3 - X-ray crystal structures of ClOF3 and BrOF3 have...Ch. 3 - Make the following comparisons about the molecules...Ch. 3 - Prob. 3.20PCh. 3 - A solution containing the lO2F2 ion reacts slowly...Ch. 3 - The XeOF3 anion has been reported recently (D. S....Ch. 3 - Predict the structure of l(CF3)Cl2 . Do you expect...Ch. 3 - a. Which has the longer axial PF distance, PF2(...Ch. 3 - Prob. 3.25PCh. 3 - SeCl62,TeCl62 , and CIF6 are all octahedral, but...Ch. 3 - Prob. 3.27PCh. 3 - The thiazyldichloride ion, NSCl2- , is...Ch. 3 - Sketch the most likely structure of PCl3Br2 and...Ch. 3 - a. Are the CF3 groups in PCl3( CF3)2 more likely...Ch. 3 - Of the molecules C1SO2CH3,C1SO2CF3 , and ClSO2CCl3...Ch. 3 - Prob. 3.32PCh. 3 - Prob. 3.33PCh. 3 - Prob. 3.34PCh. 3 - Prob. 3.35PCh. 3 - Although the CF distances and the FCF bond angles...Ch. 3 - The Cl...Cl distance in CCl4 is 289 pm, and the...Ch. 3 - The FCF angle in F2CO , shown here, is 109.5°; the...Ch. 3 - Compounds in which hydrogen is the outer atom can...Ch. 3 - For each of the following bonds, indicate which...Ch. 3 - Give Lewis dot structures and shapes for the...Ch. 3 - Give Lewis dot structures and sketch the shapes...Ch. 3 - Which of the molecules in Problem 3.41 are polar?Ch. 3 - Which of the molecules in Problem 3.42 are polar?Ch. 3 - Prob. 3.45PCh. 3 - Prob. 3.46P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forwardWhat are the relationships among bond order, bond energy, and bond length? Which of these quantities can be measured?arrow_forward• identify sigma and pi bonds in a molecule and explain the difference between them.arrow_forward
- What would you expect to be the electron-group arrangement around atom A in each of the following cases? For each arrangement, give the ideal bond angle and the direction of any expected deviation:arrow_forwardWhat role does electronegativity plays in rationalizing bond angles?arrow_forwardThe VSEPR model was developed before any xenon com-pounds had been prepared. Thus, these compounds provided anexcellent test of the model’s predictive power. What would youhave predicted for the shapes of XeF₂, XeF₄, and XeF₆?arrow_forward
- What would you expect to be the electron-group arrangement around atom A in the following case? Give the ideal bond angle. Y X A- -X Electron-group arrangement: trigonal planar trigonal bipyramidal square planar Ideal bond angle:arrow_forwardConsider the Lewis Structure for the molecule, SeF4. On your own, make a sketch of the VSEPR shape, and use that to answer the following questions: :F: F-Se-F: :F: Part A What is the electronic geometry of this molecule? O trigonal bipyramidal O t-shaped O trigonal pyramidal O tetrahedral O square planar see-saw O octahedral O O Oarrow_forwardUsing hybridization and the long method for determining the Lewis Structures, show the most feasible representation of the following compounds: AsCl4+ OClF4- (Cl is the central atom) O3BrF (Br is the central atom)arrow_forward
- What is the possible identity of X for a molecule XCl3- with a molecular geometry of trigonal pyramidal?arrow_forwardWhich theory have been given to explain shapes of the molecules. On the basis of its various postulates explain: (i) From the given examples of SF4, BF4', XeF4, IC14', the number of species having two lone pairs of electrons (ii) The bond distances and angles in SO2C12 and S2C12arrow_forwardEstimate the percent ionic character of the bond in each of the following species. All the species are unstable or reac- tive under ordinary laboratory conditions, but they can be observed in interstellar space. Bond Length (À) Dipole Moment (D) OH 0.980 1.66 CH 1.131 1.46 CN 1.175 1.45 1.246arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY