
Concept explainers
Stanozolol is an anabolic steroid that promotes muscle growth. Although stanozolol has been used by athletes and body builders, many physical and psychological problems result from prolonged use and it is banned in competitive sports.
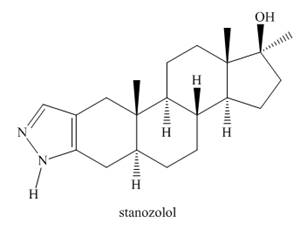
a. Explain why the nitrogen heterocycle—a pyrazole ring—is aromatic.
b. In what type of orbital is the lone pair on each
c. Draw all reasonable resonance structures for stanozolol.
d. Explain why the
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry
- a. What is the value of n in Hückel’s rule when a compound has nine pairs of p electrons? b. Is such a compound aromatic?arrow_forwardWhich of the following statements is incorrect: aromatic compounds... a. Are cyclic b. Are generally less reactive than similarly substituted alkenes c. Are planar O d. Have 4n n-electrons Benzene or the other aromatic species all have high "pi" electron density inside the aromatic ring. Select one: True O Falsearrow_forward1.Discuss how aromaticity is affected in large ring compounds. 2. Discuss the aromaticity of heterocyclic compounds. 3. Discuss the aromaticity of polynuclear hydrocarbonsarrow_forward
- Which of the following best describe the unique characteristic of aromatic compounds? a. Their high reactivity b. Their high stability c. Their toxicity d. Their unique structure.arrow_forwardF. F C. 2. F F draw structurearrow_forwarda. Consider the structure drawn below. Assuming the structure is planar, would you predict this compound to be aromatic, anti-aromatic, or non- aromatic? (Note: each nitrogen has a lone pair and, as drawn, each gallium has 6 valence electrons.) Ga .CI Ga CI b. Explain your answer for part a: Which rules of aromaticity does the compound obey or disobey?arrow_forward
- 41. Draw the structure of a hydrocarbon that has six carbon atoms and a. three vinylic hydrogens and two allylic hydrogens. b. three vinylic hydrogens and one allylic hydrogen. c. three vinylic hydrogens and no allylic hydrogens.arrow_forwardWhich molecules below are aromatic? A. B. Structure D Structure B Structure A Structure C Structure E D. E.arrow_forwardselect two. Which statements correspond to: ESCITALOPRAM (antidepressant) a. It induces tranquility and somnolence to the patient. b. An agent that regulates re-uptake of serotonin in the brain. c. Its structure has benzonitrile, flurobenzene and a tertiary amine. d. An agent that is indicated to patients with hives and dermatitis.arrow_forward
- 1. Cite some reactions in which formaldehyde behaves differently from other aldehydes. 2. Give some biological and medical applications of: a. formalin b. chloral c. urotropinearrow_forward3. How do you convert the following compounds to aromatic compounds? a. b. -CI -OHarrow_forwardDraw the structure of the ff. aldehydes and ketones. a. 3-chlorobutanal 6. 8,8-dibromo -4-cthylcyclo hexanone C. 2,4-dimetnylpontanone • Draw the structure of the ff. compounds. a. oyclobutanecarboxylic acid b. 3,8-dimcthylpontancdioic acid C. 4-aminopcntanoic acid d. 2-mothylcycloheranecarooxylic acid m- chlorobėnzoic acidarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
