
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.38P
8 In Chapter 22, we will discuss a class of compounds called amino acids, so named because they contain both an amino group and a carboxyl group. Following is a structural formula for the amino
acid alanine.
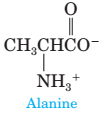
What would you expect to be the major form of alanine present in aqueous solution (a) at pH 2.0, (b) at pH 5—6, and (c) at pH 11.0? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the equation to show how pyridine, C5H5N, acts as a Brønsted-Lowry base in water.
equation: C5H5N + H2O
Name two techniques that can be employed to extract oil from seeds Hence,Name four processes that may be used to refine oils,What is the description for the term rancidity of oils? ,Name two causes of rancidity in oils, What is acid value of oils? Therefore How many moles of KOH is required per mole of fat in saponification reactions?
Compounds like amphetamine that contain nitrogen atoms are protonated by the HCl in the gastric juices of the stomach, and the resulting salt is then deprotonated in the basic environment of the intestines to regenerate the neutral form. Write proton transfer reactions for both of these processes.
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1PCh. 19.4 - Problem 19-2 Complete the equation for each...Ch. 19.4 - Prob. 19.3PCh. 19 - Prob. 19.4PCh. 19 - Write the IUPAC name for each compound.Ch. 19 - Prob. 19.6PCh. 19 - Prob. 19.7PCh. 19 - Prob. 19.8PCh. 19 - Prob. 19.9PCh. 19 - 0 Complete the equations for these reactions.
Ch. 19 - Prob. 19.11PCh. 19 - Prob. 19.12PCh. 19 - Prob. 19.13PCh. 19 - Prob. 19.14PCh. 19 - Prob. 19.15PCh. 19 - 6 Why are Dacron and Mylar referred to as...Ch. 19 - 7 What type of structural feature do the...Ch. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - 0 Show how triphosphoric acid can form from three...Ch. 19 - 1 Write an equation for the hydrolysis of...Ch. 19 - 2 (Chemical Connections 19A) Locate the ester...Ch. 19 - Prob. 19.23PCh. 19 - Prob. 19.24PCh. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - 8 (Chemical Connections 19C) Once it has been...Ch. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Prob. 19.31PCh. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - 4 (Chemical Connections 19F) Why do Lactomer...Ch. 19 - Prob. 19.35PCh. 19 - Prob. 19.36PCh. 19 - Prob. 19.37PCh. 19 - 8 In Chapter 22, we will discuss a class of...Ch. 19 - Prob. 19.39PCh. 19 - Prob. 19.40PCh. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the equation to show how pyridine, C, H, N, acts as a Brønsted-Lowry base in water. equation: C₂H₂N+H₂O=arrow_forward2. Will a 0.10 molar solution of CH3NH3OCN (methylamine cyanate) produce a basic or acidic solution. Explain quantitatively.arrow_forwardAn analytical chemist is titrating 147.6 mL of a 0.4800M solution of ethylamine (C₂H5NH₂) with a 0.2200M solution of HNO3. The pK, of ethylamine is 3.19. Calculate the pH of the base solution after the chemist has added 202.2 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = 0 Xarrow_forward
- 2-methylvaleric acid, CH3(CH2)2CH(CH3)COOH, has a pKa=4.9. draw the skeletal structure of the conjugate base of 2-methylvakeric acid and give the pH above which 90% of the compound will be in this conjugate base form.arrow_forwardThe antimalaria properties of quinine (C20H24N202) saved thousands of lives during construction of the Panama Canal. This substance is a classic example of the medicinal wealth that tropical forests hold. Both N atoms are basic, and the N of the amine group is far more basic (pKb=4.94) than the N within the aromatic ring system (pKb=10.54). A saturated solution of quinine in water is only 4.55x10-3M. What is the pH of this solution? Record your answer with 2 decimals.arrow_forwardCompounds like amphetamine that contain nitrogen atoms are protonated by the HCl in the gastric juices of the stomach, and the resulting salt is then deprotonated in the basic environment of the intestines to regenerate the neutral form. Write proton transfer reactions for both of these processes. In which form will amphetamine pass through a cell membrane?arrow_forward
- An analytical chemist is titrating 59.0 mL of a 0.8100M solution of ethylamine (C₂H₂NH₂) with a 0.4700M solution of HNO3. The pK, of ethylamine is 3.19. Calculate the pH of the base solution after the chemist has added 74.6 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. Round your answer to 2 decimal places.arrow_forwardYou have an organic acid, organic base, and a neutral organic compound all together in a solid mixture. What steps would you take, in order, to isolate the organic acid?arrow_forwardDraw the structures of aspirin, caffeine, and acetaminophen. Give the hybridization of each carbon atom and state which is acidic, weakly acidic, and basic. Identify the sites on each compound that make each acidic, weakly acidic, and basic.arrow_forward
- Answer the following statement. Provide an explanation. What happens to compound X when ph=pkb ph>pkb pharrow_forwardWrite the products of the reaction of diphenhydramine (a base) with the acid HCl shown below. Are the reactants or products more soluble in water? Briefly explain.arrow_forwardPart A The chemical 5-amino-2,3-dihydro-1,4-phthalazinedione, better known as luminol, is used by forensic scientists in analyzing crime scenes for the presence of washed-away blood. Luminol is so sensitive that it can detect blood that has been diluted 10,000 times. A basic solution of luminol is often sprayed onto surfaces that are suspected of containing minute amounts of blood. The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H2O. What is the molarity of the stock solution of luminol? Express your answer with the appropriate units. Luminol has a molecular weight of 177 g/mol. • View Available Hint(s) HẢ molarity of luminol solution = Value Units Submit Part B Before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00x10-2 M. The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY