
(a)
Interpretation:
The
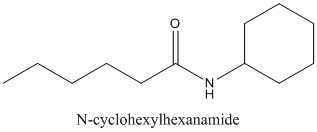
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
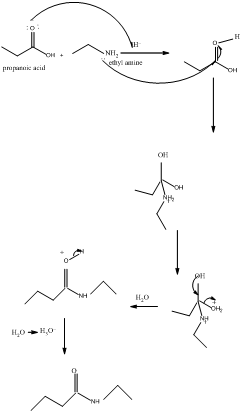
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
(b)
Interpretation:
The carboxylic acid and amine or ammonia needs to be identified from which each amide be synthesized.
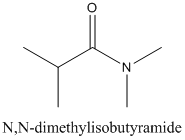
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
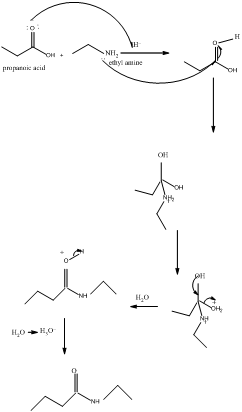
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
(c)
Interpretation:
The carboxylic acid and amine or ammonia needs to be identified from which each amide be synthesized.
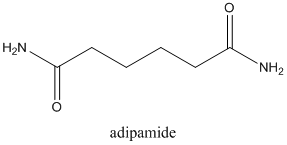
Concept Introduction:
Amides are formed by a reaction with a carboxylic acid and an amine which are the functional groups. This results in the formation of amide bond, in which OH group of carboxylic acid reacts with one of the H in amine group and CO-NH bond is formed. This bond is known as an amide bond.
The mechanism for formation of an amide is as follows taking example of propanoic acid and ethylamine:
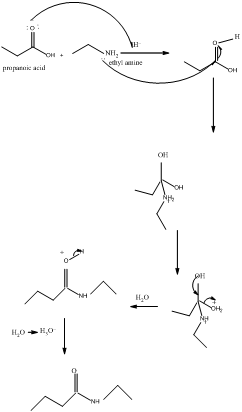
The hydrolysis of amide so formed can give back the carboxylic acid and amine.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
- Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. What functional group Erythronolide B does contain? a. b. H₂CH₂C C. H₂C 1 H₂C 2 3 4 O Amide d. Amine OH Erythronolide B Ketone Aldehyde a CH₂ b C d CH₂ OH JCH₂ 'OH OHarrow_forwardGive each of the following amines an IUPAC name. a. b. c.arrow_forwardWhat amide(s) can be used to prepare each amine by reduction?arrow_forward
- Using the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forward454 | Chapter 15 Amines (a) Which of the two nitrogen atoms in epibatadine is 54 the stronger base? (b) Mark the three stereocenters in this molecule. Cl H N Narrow_forwardWhen hexanoic acid (a carboxlic acid) is heated, a reaction takes place that generates water (which must be removed) and a non-acidic product. What is the product? carboxylate carboxylic acid anhydride ester amide lies! all lies! no reaction will take place!arrow_forward
- 11. Write the chemical reaction for the acid hydrolysis of each amide sample. Include the 유 condensed structural formulas of the reactants and products. 11 CH3-C-NH₂ +H20+HCI CH3-C-OH + NH4+ CI- ACETAMIDE SO -NH2 +H20 T → 0 COOH + NH 3 11arrow_forwardAmines as Bases. Explain ?arrow_forwardNaming Amides Named as a derivative of the carboxylic acid Take carboxylic acid name, remove-ic acid Add-amide to common name or IUPAC H-C-OH Formic acid (methanoic acid) O H–C—NH, Formamide (methanamide) 2 O NH₂arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


