
(a)
The moment of inertia and rotation energy.
(a)
Answer to Problem 31P
The moment of inertia is
Explanation of Solution
Given:
The equilibrium separation is
Formula used:
The expression for moment of inertia is given by,
The expression for rotational energy is given by,
Calculation:
The moment of inertiais calculated as,
The rotational energy is calculated as,
Conclusion:
Therefore, the moment of inertia is
(b)
The energy level diagram.
(b)
Answer to Problem 31P
The energy level diagram is shown in figure 1.
Explanation of Solution
Calculation:
The energy level diagram for the rotational level from
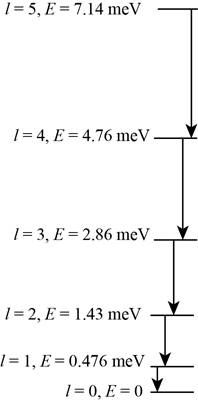
Figure 1
Conclusion:
Therefore, the energy level diagram is shown in figure 1.
(c)
The wavelength for each transition of part (b).
(c)
Answer to Problem 31P
The wavelengths for each transition from start are
Explanation of Solution
Formula used:
The expression for wavelength is given by,
Calculation:
The first wavelength is calculated as,
The second wavelength is calculated as,
The third wavelength is calculated as,
The fourth wavelength is calculated as,
The fifth wavelength is calculated as,
Conclusion:
Therefore, the wavelengths for each transition from start are
Want to see more full solutions like this?
Chapter 37 Solutions
Physics for Scientists and Engineers
- Consider the HCl molecule, which consists of a hydrogen atom of mass 1 u bound to a chlorine atom of mass 35 u. The equilibrium separation between the atoms is 0.128 nm, and it requires 0.15 eV of work to increase or decrease this separation by 0.01 nm. (a) Calculate the four lowest rotational energies (in eV) that are possible, assuming the molecule rotates rigidly. (b) Find the molecules spring constant and its classical frequency of vibration. (Hint: Recall that U=12Kx2.) (c) Find the two lowest vibrational energies and the classical amplitude of oscillation corresponding to each of these energies. (d) Determine the longest wavelength radiation that the molecule can emit in a pure rotational transition and in a pure vibrational transition.arrow_forwardThe CO molecule makes a transition from the J = 1 to the J = 2 rotational state when it absorbs a photon of frequency 2.30 x 1011 Hz. (a) Find the moment of inertia of this molecule from these data.arrow_forwardThe frequency of the photon that causes the υ = 0 to υ = 1 transition in the CO molecule is 6.42 x 1013 Hz. We ignore any changes in the rotational energy for this example.(A) Calculate the force constant k for this molecule. (B) What is the classical amplitude A of vibration for this molecule in the υ = 0 vibrational state?arrow_forward
- What is the energy of a photon emitted from a CO molecule that transitions from the rotational Ji to Ji-1 level. Express your answer in units of ℏ^2/I, so you just have an integer value as a final answer. Values: Ji = 3 The equation I have in the teacher's ppt is E_rotational = (J*(J+1)*ℏ^2)/(2*I)arrow_forwardThis problem deals with the splitting of rotational energy levels of diatomic molecules. If one atom of the molecule has more than one stable isotope, then both isotopes are normally present in a sample. Show that the fractional change ∆f/f in the observed frequency of a photon emitted in a transition between adjacent rotational states is equal to the fractional difference in the reduced mass ∆μ/μ for molecules containing the two different isotopes.arrow_forwardIn a vibrational-rotational spectroscopy the total energy is the sum of the energies coming from the vibration and rotation (E = E + E₁). Selection rule suggests that for transition to occur Av = ±1 and Al = ±1. At room temperature, it is assumed that the lowest vibrational state is populated and the energy can only travel upwards due to lack of population of upper vibrational states thus Av = +1. What would be the energy of a line for R, P and Q-branch if a.) Al = +1, b.) Al = -1 and c.) Al = 0 respectively.arrow_forward
- The effective spring constant associated with bonding in the N2 molecule is 2 297 N/m. The nitrogen atoms each have a mass of 2.32 x 10-26 kg, and their nuclei are 0.120 nm apart. Assume the molecule is rigid. The first excited vibrational state of the molecule is above the vibrational ground state by an energy difference ΔE. Calculate the J value of the rotational state that is above the rotational ground state by the same energy difference ΔE.arrow_forwardConsider a CO molecule that is initially in the ground state of n = 0, l = 0. If the energy of a vibrational transition from the n = 0 state to the n = 1 state in CO could instead be absorbed in a rotational transition, what would be the value of l for the final state?arrow_forwardWe can apply the rigid rotor approximation to the rotational energy levels of a diatomic molecule, with E, = BJ(J + 1), where B represents a rotational constant. (a) Given the selection rules for ro-vibrational transitions, develop a formula for an absorption in the R branch from a state with angular momentum J. Assume a harmonic oscillator vibration with frequency 785 cm¹ originating in the ground vibrational state (i.e. n = 0). You will be given a value for B and asked to compute the energy for a particular transition on the submission quiz. (b) Suppose you measure several R-branch rotational transitions for 24Mg¹60, and you find that the absorption lines are uniformly spaced 1.1485 cm-¹ apart. The quantity, B, represents a rotational constant related to the moment of inertia, I, of the molecule, via h B = 8π² cl when expressed in wavenumber (cm-¹) units. Based on this information, what is the value of the moment of inertia (in kg-m²) for MgO? (c) Using the results you have found,…arrow_forward
- In solid KCI the smallest distance between the centers of a. potassium ion and a chloride ion is 314 pm. Calculate the length of the edge of the unit cell and the density of KCI, assuming it has the same structure as sodium chloride.arrow_forwardThe moment of inertia of water molecule about an axis bisecting the HOH angle is1.91x10-47 kg m2. Its minimum angular momentum about that axis (other than zero) is ℏ. Inclassical terms, how many revolutions per second do the hydrogen atoms make about the axiswhen in that state? Calculate the rotational constant (cm-1) and bond length of H2O. Does the bondlength seem reasonable?arrow_forwardThe diatomic molecule N2 has a rotational constant B(~) = 2.0 cm-1 and a vibrational constant v(~) = 2400 cm-1. The symmetry number for the molecule is 2. Sorry that I cannot write the symbols properly here for the wavenumber versions of the spectroscopic constants. (a) Calculate the rotational partition function and the vibrational partition function for N2 at T = 298 K assuming the high-temperature limit is valid in both cases. (b) Suppose that a high-temperature limit for a partition function gives the value q = 0.34. Comment on the value and whether the high-temperature limit is valid.arrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

