
Concept explainers
a)
Interpretation:
The carbocation has to be arranged in increasing order.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon. Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation as shown below.
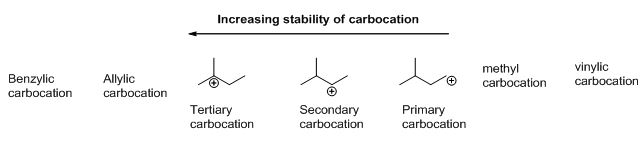
b)
Interpretation:
The carbocation has to be arranged in increasing order.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depending on the stability of the carbocation. This type of carbocation undergoes inter or intra molecule reactions and it form more stable product this type of rearrangement called carbocation rearrangement.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon. Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation as shown below.
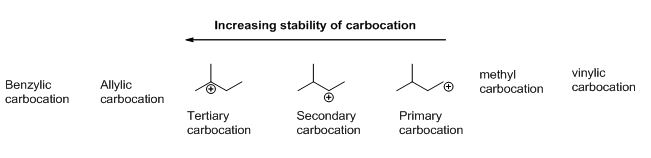
c)
Interpretation:
The carbocation has to be arranged in increasing order.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depending on the stability of the carbocation. This type of carbocation undergoes inter or intra molecule reactions and it form more stable product this type of rearrangement called carbocation rearrangement.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon. Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation as shown below.
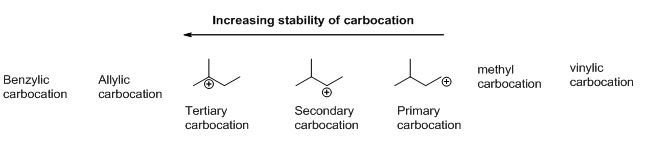
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry
- Rank the alkenes shown in the ball-and-stick models (A-C) in order of increasing stability. Barrow_forwardArrange the following radicals in order of increasing stability. A B C D Earrow_forwardOrder these according to decreasing stability; 1 being the most stable, 4 the least stable. A B C Darrow_forward
- Arrange the following carbocations in order of increasing stability. Explain your answer. a) b) CH3 H3C-C-CH₂ H H CH3 + 3 H₂C-C-CH₂CH₂ CH₂ H,C-C-CH₂CH₂ H3C-C-CH3 T CH 3 & Öd CH3CH2 j H H3C- CH 3arrow_forwardArrange the following compounds in the increasing order of reactivity towards Conc.HNO3 & Conc.H2SO4 1. Benzene 2. Chlorobenzene 3. phenol 4. Toluene 5. Nitrobenzene A 1,2,3,4,5 B) 5,2,1,4,3 C) 5,1,4,2,3 D) 5,1,2,4,3arrow_forwardArrange the following according to INCREASING reactivity towards E2: 1st ( least reactive)? 2nd? 3rd? 4th (most reactive)?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





